Journal of CO2 Utilization ( IF 7.7 ) Pub Date : 2021-06-10 , DOI: 10.1016/j.jcou.2021.101605 Theodoros Papalas , Iakovos Polychronidis , Andy N. Antzaras , Angeliki A. Lemonidou
|
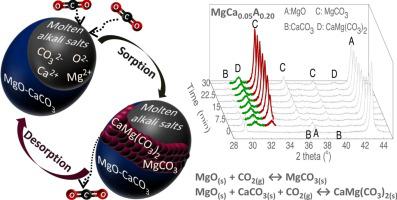
Carbonate looping using MgO-based sorbents has recently aroused the scientific interest as an auspicious technology for intermediate temperature (200−400 °C) CO2 capture, with the main hindrance being the slow sorption kinetics of MgO. This study investigated the preparation of MgO-based sorbents derived from mineral magnesite and promoted with a mixture of Li, Na and K nitrates, which by shifting into a molten state can reinforce the CO2 capture kinetics. Mineral limestone was also tested as promoter, which enabled the formation of CaMg(CO3)2 as another carbonate product except MgCO3. In-situ X-ray diffraction was employed to interpret the sorption mechanism of materials with different promoter contents and under various operating conditions. Even though CaMg(CO3)2 required a minimum temperature of 300 °C for formation in contrast to MgCO3, it generally displayed a faster nucleation rate, which was profitable mainly when applying a low CaCO3 to MgO molar ratio. However, the CaMg(CO3)2 decomposition demanded higher temperatures than MgCO3, which were associated with more pronounced sintering of the sorbent and elevated energy requirements of the process. The increase of the alkali nitrate content led to a deterioration of the pore network, but also enabled a faster generation and growth of carbonate products. Lowering the CO2 concentration of gas feedstock for more realistic carbonate looping applications required a slightly milder operating temperature for efficient CO2 sorption to enhance both CO2 solubility and carbonation kinetic driving force. The results proved the feasibility of mineral ores as sustainable low-cost precursors for intermediate-temperature CO2 capture.
中文翻译:

通过熔融碱金属硝酸盐和 CaCO 3提高矿物 MgO的中温 CO 2捕获效率:表征和吸附机制
使用基于 MgO 的吸附剂的碳酸盐循环最近引起了科学兴趣,作为一种用于中温 (200-400 °C) CO 2捕获的吉祥技术,主要障碍是 MgO 的缓慢吸附动力学。本研究调查了从菱镁矿中提取的 MgO 基吸附剂的制备,并用 Li、Na 和 K 硝酸盐的混合物促进,通过转变为熔融状态可以增强 CO 2捕获动力学。还测试了矿物石灰石作为促进剂,其能够形成 CaMg(CO 3 ) 2作为除 MgCO 3之外的另一种碳酸盐产物。原位X射线衍射被用来解释不同助剂含量和不同操作条件下材料的吸附机理。尽管与 MgCO 3相比,CaMg(CO 3 ) 2 的形成需要 300 °C 的最低温度,但它通常显示出更快的成核速率,这主要在应用低 CaCO 3与 MgO 摩尔比时有利。然而,CaMg(CO 3 ) 2分解需要比 MgCO 3更高的温度,这与更显着的吸附剂烧结和过程的能量需求增加有关。碱金属硝酸盐含量的增加导致孔隙网络的恶化,但也使碳酸盐产物的生成和生长更快。为更现实的碳酸盐循环应用降低气体原料的 CO 2浓度需要稍微温和的操作温度以有效吸收CO 2以提高 CO 2溶解度和碳酸化动力学驱动力。结果证明了矿物矿石作为中温 CO 2捕获的可持续低成本前体的可行性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号