当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Biaryl Diols via Dynamic Kinetic Resolution
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2021-06-09 , DOI: 10.1002/bkcs.12337 Jeonghun Cho 1 , Kyungwoo Kim 1 , Jaiwook Park 1 , Mahn‐Joo Kim 1
Bulletin of the Korean Chemical Society ( IF 1.7 ) Pub Date : 2021-06-09 , DOI: 10.1002/bkcs.12337 Jeonghun Cho 1 , Kyungwoo Kim 1 , Jaiwook Park 1 , Mahn‐Joo Kim 1
Affiliation
|
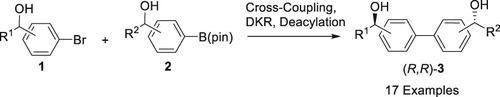
We have developed a protocol incuding dynamic kinetic resolution as the key step for the asymmetric synthesis of biaryl diol stereoisomers. The two aryl alkanols as the starting materials were cross-coupled by palladium catalysis to give dl- and meso-diol, which were then subject to chemoenzymatic dynamic kinetic resolution (DKR) for the transformation into single stereoisomeric diol diester. Diol diester was finally deacylated to give the corresponding biaryl diol stereoisomer. The chemoenzymatic DKR was performed with a ruthenium-based racemization catalyst and a (R)-selective lipoprotein lipase. Total 17 (R,R)-diols including 7 C2-symmetric diols were synthesized with excellent enantiopurities (>99% ee).
中文翻译:

通过动态动力学拆分不对称合成联芳二醇
我们开发了一个协议,包括动态动力学分辨率作为联芳二醇立体异构体不对称合成的关键步骤。两个芳基链烷醇作为起始材料是交叉耦合的由钯催化,得到DL -和内消旋-二醇,然后将其经受化学酶促动态动力学拆分( DKR)用于转化成单立体异构体的二醇二酯。二醇二酯最终脱酰化得到相应的联芳二醇立体异构体。化学酶促 DKR 使用基于钌的外消旋化催化剂和 ( R ) 选择性脂蛋白脂肪酶进行。总共 17 ( R , R )-二醇,包括 7 C 2合成了具有优异对映纯度 (>99% ee ) 的 -对称二醇。
更新日期:2021-07-27
中文翻译:

通过动态动力学拆分不对称合成联芳二醇
我们开发了一个协议,包括动态动力学分辨率作为联芳二醇立体异构体不对称合成的关键步骤。两个芳基链烷醇作为起始材料是交叉耦合的由钯催化,得到DL -和内消旋-二醇,然后将其经受化学酶促动态动力学拆分( DKR)用于转化成单立体异构体的二醇二酯。二醇二酯最终脱酰化得到相应的联芳二醇立体异构体。化学酶促 DKR 使用基于钌的外消旋化催化剂和 ( R ) 选择性脂蛋白脂肪酶进行。总共 17 ( R , R )-二醇,包括 7 C 2合成了具有优异对映纯度 (>99% ee ) 的 -对称二醇。

























 京公网安备 11010802027423号
京公网安备 11010802027423号