Journal of Cystic Fibrosis ( IF 5.2 ) Pub Date : 2021-06-08 , DOI: 10.1016/j.jcf.2021.04.012 Anne Bertelsen 1 , J Stuart Elborn 2 , Bettina C Schock 3
|
Background
In Cystic Fibrosis (CF) airways, the dehydrated, thick mucus promotes the establishment of persistent polymicrobial infections and drives chronic airways inflammation. This also predisposes the airways to further infections, the vicious, self-perpetuating cycle causing lung damage and progressive lung function decline. The airways are a poly-microbial environment, containing both aerobic and anaerobic bacterial species. Pseudomonas aeruginosa (P. aeruginosa) infections contribute to the excessive inflammatory response in CF, but the role of anaerobic Prevotella spp., frequently found in CF airways, is not known.
Materials
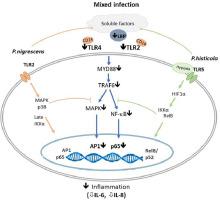
We assessed innate immune signalling in CF airway epithelial cells in response to clinical strains of P. histicola, P. nigresens and P. aeruginosa. CFBE41o- cells were infected with P. aeruginosa (MOI 100, 2h) followed by infection with P. histicola or P. nigrescens (MOI 100, 2h). Cells were incubated under anaerobic conditions for the duration of the experiments.
Results
Our study shows that P. histicola and P. nigresens can reduce the growth of P. aeruginosa and dampen the inflammatory response in airway epithelial cells. We specifically illustrate that the presence of the investigated Prevotella spp. reduces Toll-like-receptor (TLR)-4, MAPK, NF-κB(p65) signalling and cytokine release (Interleukin (IL)-6, IL-8) in mixed infections.
Conclusion
Our work, for the first time, strongly indicates a relationship between P. aeruginosa and anaerobic Prevotella spp.. The observed modified NF-κB and MAPK signalling indicates some mechanisms underlying this interaction that could offer a novel therapeutic approach to combat chronic P. aeruginosa infection in people with CF.
中文翻译:

微生物相互作用:普氏菌属。减少铜绿假单胞菌诱导的囊性纤维化支气管上皮细胞炎症
背景
在囊性纤维化 (CF) 气道中,脱水的粘稠粘液会促进持续性多种微生物感染的形成,并导致慢性气道炎症。这也使气道容易受到进一步感染,恶性循环导致肺损伤和进行性肺功能下降。气道是一个多微生物环境,包含需氧和厌氧细菌种类。铜绿假单胞菌( P. aeruginosa)感染导致 CF 中的过度炎症反应,但在 CF 气道中常见的厌氧普氏菌的作用尚不清楚。
材料
我们评估了 CF 气道上皮细胞对P. histicola、P. nigresens和P. aeruginosa临床菌株的反应中的先天免疫信号传导。CFBE41o-细胞用铜绿假单胞菌感染(MOI 100, 2h),然后用P. histicola或P. nigrescens感染(MOI 100, 2h)。在实验期间,细胞在厌氧条件下孵育。
结果
我们的研究表明P. histicola 和 P. nigresens可以减少P. aeruginosa的生长并抑制气道上皮细胞的炎症反应。我们特别说明了调查的Prevotella spp 的存在。减少混合感染中的 Toll 样受体 (TLR)-4、MAPK、NF-κB(p65) 信号传导和细胞因子释放(白细胞介素 (IL)-6、IL-8)。
结论
我们的工作首次有力地表明了铜绿假单胞菌和厌氧普氏菌之间的关系。观察到的修饰的 NF-κB 和 MAPK 信号表明这种相互作用背后的一些机制可以提供一种新的治疗方法来对抗慢性铜绿假单胞菌CF 患者感染。


























 京公网安备 11010802027423号
京公网安备 11010802027423号