Cell Calcium ( IF 4 ) Pub Date : 2021-06-08 , DOI: 10.1016/j.ceca.2021.102432 A Shalygin 1 , D Kolesnikov 1 , L Glushankova 1 , K Gusev 1 , A Skopin 1 , K Skobeleva 1 , E V Kaznacheyeva 1
|
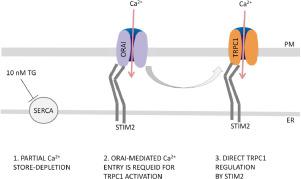
Store-operated calcium channels are the major player in calcium signaling in non-excitable cells. Store-operated calcium entry is associated with the Orai, stromal interaction molecule (STIM), and transient receptor potential canonical (TRPC) protein families. Researchers have provided conflicting data about TRPC1 channel regulation by Orai and STIM. To determine how Orai and STIM influence endogenous TRPC1 pore properties and regulation, we used single channel patch-clamp recordings. Here we showed that knockout or knockdown of Orai1 or Orai3 or overexpression of the dominant-negative mutant Orai1 E106Q did not change the conductance or selectivity of single TRPC1 channels. In addition, these TRPC1 channel properties did not depend on the amount of STIM1 and STIM2 proteins. To study STIM2-mediated regulation of TRPC1 channels, we utilized partial calcium store depletion induced by application of 10 nM thapsigargin (Tg). TRPC1 activation by endogenous STIM2 was greatly decreased in acute extracellular calcium-free experiments. STIM2 overexpression increased both the basal activity and number of silent TRPC1 channels in the plasma membrane. After calcium store depletion, overexpressed STIM2 directly activated TRPC1 in the plasma membrane even without calcium entry in acute experiments. However, this effect was abrogated by co-expression with the non-permeable Orai1 E106Q mutant protein. Taken together, our single-channel patch clamp experiments clearly demonstrated that endogenous TRPC1 forms a channel pore without involving Orai proteins. Calcium entry through Orai triggered TRPC1 channel activation in the plasma membrane, while subsequent STIM2-mediated TRPC1 activity regulation was not dependent on calcium entry.
中文翻译:

STIM2和Orai蛋白在钙库耗竭后调节TRPC1通道活性中的作用
存储操作的钙通道是非兴奋细胞中钙信号传导的主要参与者。钙库操作的钙进入与 Orai、基质相互作用分子 (STIM) 和瞬时受体电位规范 (TRPC) 蛋白家族相关。研究人员提供了有关 Orai 和 STIM 对 TRPC1 通道调节的相互矛盾的数据。为了确定 Orai 和 STIM 如何影响内源性 TRPC1 孔特性和调节,我们使用了单通道膜片钳记录。在这里,我们表明敲除或敲除 Orai1 或 Orai3 或显性阴性突变体 Orai1 E106Q 的过表达不会改变单个 TRPC1 通道的电导或选择性。此外,这些 TRPC1 通道特性不依赖于 STIM1 和 STIM2 蛋白的数量。为了研究 STIM2 介导的 TRPC1 通道调节,我们利用由应用 10 nM 毒胡萝卜素 (Tg) 引起的部分钙库耗竭。在急性细胞外无钙实验中,内源性 STIM2 对 TRPC1 的激活大大降低。STIM2 过表达增加了质膜中的基础活性和沉默 TRPC1 通道的数量。在钙储备耗尽后,即使在急性实验中没有钙进入,过表达的 STIM2 也会直接激活质膜中的 TRPC1。然而,通过与不可渗透的 Orai1 E106Q 突变蛋白共表达,这种效果被取消。总之,我们的单通道膜片钳实验清楚地表明,内源性 TRPC1 形成了一个通道孔,而不涉及 Orai 蛋白。钙通过 Orai 进入,触发质膜中的 TRPC1 通道激活,



























 京公网安备 11010802027423号
京公网安备 11010802027423号