当前位置:
X-MOL 学术
›
RSC Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deciphering the substrate recognition mechanisms of the heparan sulfate 3-O-sulfotransferase-3
RSC Chemical Biology Pub Date : 2021-5-28 , DOI: 10.1039/d1cb00079a Rylee Wander 1 , Andrea M Kaminski 2 , Yongmei Xu 1 , Vijayakanth Pagadala 3 , Juno M Krahn 2 , Truong Quang Pham 3 , Jian Liu 1 , Lars C Pedersen 2
RSC Chemical Biology Pub Date : 2021-5-28 , DOI: 10.1039/d1cb00079a Rylee Wander 1 , Andrea M Kaminski 2 , Yongmei Xu 1 , Vijayakanth Pagadala 3 , Juno M Krahn 2 , Truong Quang Pham 3 , Jian Liu 1 , Lars C Pedersen 2
Affiliation
|
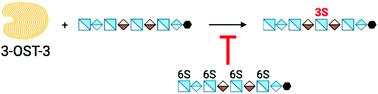
The sulfation at the 3-OH position of a glucosamine saccharide is a rare modification, but is critically important for the biological activities of heparan sulfate polysaccharides. Heparan sulfate 3-O-sulfotransferase (3-OST), the enzyme responsible for completing this modification, is present in seven different isoforms in humans. Individual isoforms display substrate selectivity to uniquely sulfated saccharide sequences present in heparan sulfate polysaccharides. Here, we report two ternary crystal structures of heparan sulfate 3-OST isoform 3 (3-OST-3) with PAP (3′-phosphoadenosine 5′-phosphate) and two octasaccharide substrates: non 6-O-sulfated octasaccharide (8-mer 1) and 6-O-sulfated octasaccharide (8-mer 3). The 8-mer 1 is a known favorable substrate for 3-OST-3, whereas the 8-mer 3 is an unfavorable one. Unlike the 8-mer 1, we discovered that the 8-mer 3 displays two binding orientations to the enzyme: productive binding and non-productive binding. Results from the enzyme activity studies demonstrate that 8-mer 3 can contribute to either substrate or product inhibition, possibly attributed to a non-productive binding mode. Our results suggest that heparan sulfate substrates interact with the 3-OST-3 enzyme in more than one orientation, which may regulate the activity of the enzyme. Our findings also suggest that different binding orientations between polysaccharides and their protein binding partners could influence biological outcomes.
中文翻译:

破译硫酸乙酰肝素 3-O-sulfotransferase-3 的底物识别机制
氨基葡萄糖糖类 3-OH 位置的硫酸化是一种罕见的修饰,但对于硫酸乙酰肝素多糖的生物活性至关重要。硫酸乙酰肝素 3- O-磺基转移酶 (3-OST) 是负责完成这种修饰的酶,在人体中存在于七种不同的亚型中。单个亚型显示出对硫酸乙酰肝素多糖中存在的独特硫酸化糖序列的底物选择性。在这里,我们报告了硫酸乙酰肝素 3-OST 亚型 3 (3-OST-3) 与 PAP(3'-磷酸腺苷 5'-磷酸)和两种八糖底物的两种三元晶体结构:非 6- O-硫酸化八糖( 8- mer 1) 和 6- O-硫酸化八糖(8-mer 3)。8 聚体 1 是 3-OST-3 已知的有利底物,而 8 聚体 3 是不利底物。与 8 聚体 1 不同,我们发现 8 聚体 3 显示出与酶的两种结合方向:生产性结合和非生产性结合。酶活性研究的结果表明,8 聚体 3 可能有助于抑制底物或产物,这可能归因于非生产性结合模式。我们的结果表明,硫酸乙酰肝素底物以不止一个方向与 3-OST-3 酶相互作用,这可能调节酶的活性。我们的研究结果还表明,多糖及其蛋白质结合伙伴之间的不同结合方向可能会影响生物学结果。
更新日期:2021-06-08
中文翻译:

破译硫酸乙酰肝素 3-O-sulfotransferase-3 的底物识别机制
氨基葡萄糖糖类 3-OH 位置的硫酸化是一种罕见的修饰,但对于硫酸乙酰肝素多糖的生物活性至关重要。硫酸乙酰肝素 3- O-磺基转移酶 (3-OST) 是负责完成这种修饰的酶,在人体中存在于七种不同的亚型中。单个亚型显示出对硫酸乙酰肝素多糖中存在的独特硫酸化糖序列的底物选择性。在这里,我们报告了硫酸乙酰肝素 3-OST 亚型 3 (3-OST-3) 与 PAP(3'-磷酸腺苷 5'-磷酸)和两种八糖底物的两种三元晶体结构:非 6- O-硫酸化八糖( 8- mer 1) 和 6- O-硫酸化八糖(8-mer 3)。8 聚体 1 是 3-OST-3 已知的有利底物,而 8 聚体 3 是不利底物。与 8 聚体 1 不同,我们发现 8 聚体 3 显示出与酶的两种结合方向:生产性结合和非生产性结合。酶活性研究的结果表明,8 聚体 3 可能有助于抑制底物或产物,这可能归因于非生产性结合模式。我们的结果表明,硫酸乙酰肝素底物以不止一个方向与 3-OST-3 酶相互作用,这可能调节酶的活性。我们的研究结果还表明,多糖及其蛋白质结合伙伴之间的不同结合方向可能会影响生物学结果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号