Cell Stem Cell ( IF 23.9 ) Pub Date : 2021-06-07 , DOI: 10.1016/j.stem.2021.05.007 Sixia Huang 1 , Paola Kuri 1 , Yann Aubert 1 , Megan Brewster 1 , Ning Li 2 , Olivia Farrelly 1 , Gabriella Rice 1 , Hyunjin Bae 1 , Stephen Prouty 1 , Tzvete Dentchev 1 , Wenqin Luo 3 , Brian C Capell 4 , Panteleimon Rompolas 5
|
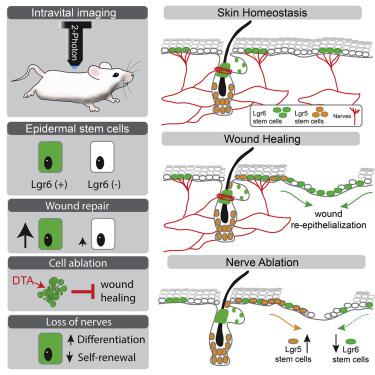
Stem cells support lifelong maintenance of adult organs, but their specific roles during injury are poorly understood. Here we demonstrate that Lgr6 marks a regionally restricted population of epidermal stem cells that interact with nerves and specialize in wound re-epithelialization. Diphtheria toxin-mediated ablation of Lgr6 stem cells delays wound healing, and skin denervation phenocopies this effect. Using intravital imaging to capture stem cell dynamics after injury, we show that wound re-epithelialization by Lgr6 stem cells is diminished following loss of nerves. This induces recruitment of other stem cell populations, including hair follicle stem cells, which partially compensate to mediate wound closure. Single-cell lineage tracing and gene expression analysis reveal that the fate of Lgr6 stem cells is shifted toward differentiation following loss of their niche. We conclude that Lgr6 epidermal stem cells are primed for injury response and interact with nerves to regulate their fate.
中文翻译:

Lgr6 标记表皮干细胞在伤口再上皮化中具有神经依赖性作用
干细胞支持成人器官的终身维护,但它们在损伤过程中的具体作用却知之甚少。在这里,我们证明 Lgr6 标志着一个区域受限的表皮干细胞群,它们与神经相互作用并专门从事伤口再上皮化。白喉毒素介导的 Lgr6 干细胞消融延迟了伤口愈合,并且皮肤去神经表型复制了这种效果。使用活体成像来捕获损伤后的干细胞动力学,我们表明 Lgr6 干细胞的伤口再上皮化在神经丧失后减少。这会诱导其他干细胞群的募集,包括毛囊干细胞,这些干细胞可以部分补偿以介导伤口闭合。单细胞谱系追踪和基因表达分析表明,Lgr6 干细胞的命运在失去其生态位后转向分化。我们得出结论,Lgr6 表皮干细胞已准备好进行损伤反应并与神经相互作用以调节其命运。


























 京公网安备 11010802027423号
京公网安备 11010802027423号