Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2021-06-07 , DOI: 10.1016/j.xcrp.2021.100455 Li Zhang , Zhenhua Zeng , Da-Wei Wang , Yalu Zuo , Jiangtao Chen , Xingbin Yan
|
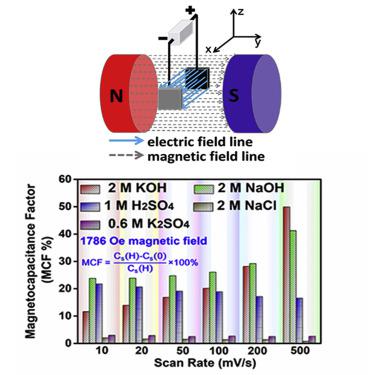
Carbon-based supercapacitors (SCs) are important electrochemical energy storage devices and are often used in electronic equipment that generates a magnetic field. However, whether the magnetic field affects the charge storage of SCs is unknown. Here, we discover that applying an external magnetic field to carbon-based SCs can induce capacitance change in both aqueous acidic and alkaline electrolytes but not in neutral electrolytes. We further show that, in KOH electrolytes, the competition between the driving force caused by the Lorentz force and the damping force related to the electrolyte conductivity plays a crucial role in affecting the OH− transport under the magnetic field. In H2SO4 electrolytes, the third paramagnetic force generated by the paramagnetic H+ under the magnetic field hinders the H+ transport in addition to the above forces. A quantitative relationship among the limiting current density at the electrode-electrolyte interface, the intensity of the magnetic field, and the concentration and viscosity of electrolytes can therefore be established.
中文翻译:

水性碳基超级电容器中磁场引起的电容变化
碳基超级电容器 (SC) 是重要的电化学储能装置,常用于产生磁场的电子设备中。然而,磁场是否影响SCs的电荷存储尚不清楚。在这里,我们发现对碳基 SCs 施加外部磁场可以引起酸性和碱性水溶液电解质的电容变化,但不会引起中性电解质的电容变化。我们进一步表明,在 KOH 电解质中,洛伦兹力引起的驱动力与与电解质电导率相关的阻尼力之间的竞争在影响磁场下OH -传输方面起着至关重要的作用。在 H 2 SO 4在电解质中,磁场下顺磁性 H +产生的第三种顺磁力阻碍了 H + 的传输。因此,可以在电极-电解质界面的极限电流密度、磁场强度以及电解质的浓度和粘度之间建立定量关系。



























 京公网安备 11010802027423号
京公网安备 11010802027423号