Journal of Structural Biology ( IF 3 ) Pub Date : 2021-06-06 , DOI: 10.1016/j.jsb.2021.107751 Jie Gao 1 , Cong Xiao 2 , Shanhui Liao 2 , Xiaoming Tu 2
|
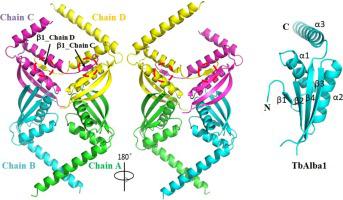
Alba (Acetylation lowers binding affinity) domain is a small, dimeric nucleic acid-binding domain, which is widely distributed in archaea and numbers of eukaryotes. Alba domain containing proteins have been reported to be involved in many cellular processes, such as regulation of translation, maintaining genome stability, regulation of RNA processing and so on. In Trypanosoma brucei (T. brucei), there are four Alba proteins identified, which are named TbAlba1 to TbAlba4. However, the structure and function of TbAlba proteins are still unknown. Here, we solved the crystal structure of TbAlba1 to a resolution of 2.46 Å. TbAlba1 adopts a similar Alba-fold, which comprises of four β-strands (β1-β4) and three long α-helices (α1-α3). Furthermore, TbAlba1 displays some structural features quite different from other Alba proteins. These differences may imply the diverse biological roles of Alba family members.
中文翻译:

布氏锥虫 TbAlba1 的晶体结构
Alba ( A cetylation lowers b inding a ffinity )结构域是一个小的二聚体核酸结合结构域,广泛分布于古细菌和大量真核生物中。据报道,含有 Alba 结构域的蛋白质参与了许多细胞过程,例如调节翻译、维持基因组稳定性、调节 RNA 加工等。在布氏锥虫 (T. brucei),鉴定出四种 Alba 蛋白,分别命名为 TbAlba1 至 TbAlba4。然而,TbAlba 蛋白的结构和功能仍然未知。在这里,我们将 TbAlba1 的晶体结构解析为 2.46 Å 的分辨率。TbAlba1 采用类似的 Alba 折叠,由四个 β-链 (β1-β4) 和三个长 α-螺旋 (α1-α3) 组成。此外,TbAlba1 显示出一些与其他 Alba 蛋白完全不同的结构特征。这些差异可能意味着阿尔巴家族成员的不同生物学角色。



























 京公网安备 11010802027423号
京公网安备 11010802027423号