当前位置:
X-MOL 学术
›
Clin. Transl. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A lymphodepleted non-human primate model for the assessment of acute on-target and off-tumor toxicity of human chimeric antigen receptor-T cells
Clinical & Translational Immunology ( IF 5.8 ) Pub Date : 2021-06-03 , DOI: 10.1002/cti2.1291 Shigeki Yagyu 1, 2 , Hidemi Mochizuki 2, 3 , Kumiko Yamashima 1, 4 , Hiroshi Kubo 1 , Shoji Saito 2, 5 , Miyuki Tanaka 2, 5 , Kengo Sakamoto 3 , Akihito Shimoi 2, 3 , Yozo Nakazawa 2, 5, 6
Clinical & Translational Immunology ( IF 5.8 ) Pub Date : 2021-06-03 , DOI: 10.1002/cti2.1291 Shigeki Yagyu 1, 2 , Hidemi Mochizuki 2, 3 , Kumiko Yamashima 1, 4 , Hiroshi Kubo 1 , Shoji Saito 2, 5 , Miyuki Tanaka 2, 5 , Kengo Sakamoto 3 , Akihito Shimoi 2, 3 , Yozo Nakazawa 2, 5, 6
Affiliation
|
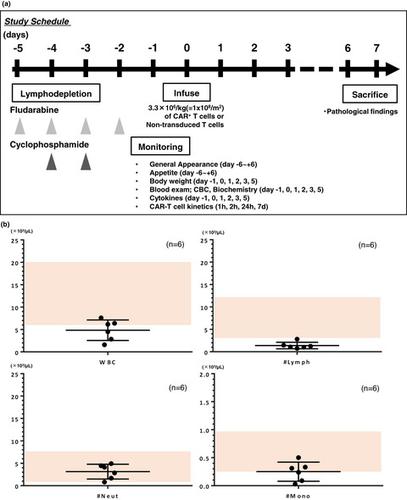
Chimeric antigen receptor (CAR)-T cell therapy possesses the potential to cause unexpected on-target toxicities that may be life-threatening. Non-human primates (NHPs) share considerable structural homology and expression profiles of most proteins with humans and are therefore utilised as an animal model for non-clinical safety studies. We have developed a lymphodepleted NHP model by conditioning the animals with immunosuppressive chemotherapy designed to simulate clinical practice conditions, to induce transient mixed chimerism before the administration of human CAR-T cells redirected to target Ephrin type-B receptor 4 (EPHB4-CAR-T cells) to evaluate the toxicity of these cells.
中文翻译:

用于评估人嵌合抗原受体 T 细胞的急性靶向和肿瘤外毒性的去淋巴细胞非人灵长类动物模型
嵌合抗原受体 (CAR)-T 细胞疗法有可能导致意想不到的靶向毒性,可能危及生命。非人类灵长类动物 (NHP) 与人类的大多数蛋白质具有相当大的结构同源性和表达谱,因此被用作非临床安全性研究的动物模型。我们开发了一种清除淋巴的 NHP 模型,通过免疫抑制化疗对动物进行调节,旨在模拟临床实践条件,在施用重定向到靶向 Ephrin B 型受体 4 (EPHB4-CAR-T) 的人类 CAR-T 细胞之前诱导瞬时混合嵌合体细胞)来评估这些细胞的毒性。
更新日期:2021-06-04
中文翻译:

用于评估人嵌合抗原受体 T 细胞的急性靶向和肿瘤外毒性的去淋巴细胞非人灵长类动物模型
嵌合抗原受体 (CAR)-T 细胞疗法有可能导致意想不到的靶向毒性,可能危及生命。非人类灵长类动物 (NHP) 与人类的大多数蛋白质具有相当大的结构同源性和表达谱,因此被用作非临床安全性研究的动物模型。我们开发了一种清除淋巴的 NHP 模型,通过免疫抑制化疗对动物进行调节,旨在模拟临床实践条件,在施用重定向到靶向 Ephrin B 型受体 4 (EPHB4-CAR-T) 的人类 CAR-T 细胞之前诱导瞬时混合嵌合体细胞)来评估这些细胞的毒性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号