Chemistry of Heterocyclic Compounds ( IF 1.5 ) Pub Date : 2021-06-04 , DOI: 10.1007/s10593-021-02944-0 Maxim R. Demidov , Anastasiya N. Dobrokvashina , Dmitry V. Osipov , Vitaly А. Osyanin , Yuri N. Klimochkin
|
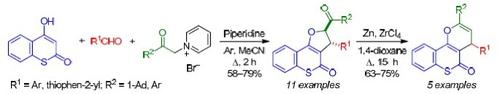
Three-component condensation of in situ generated pyridinium acyl methylides with aromatic aldehydes and 4-hydroxythiocoumarin led to a series of 2-acyl-2,3-dihydro-4H-thiochromeno[4,3-b]furan-4-ones. The reaction proceeds diastereoselectively with the formation of trans-isomers and represents a cascade process involving the Knoevenagel condensation, carbo-Michael reaction, and intramolecular nucleophilic substitution. The subsequent redox rearrangement of 2-acyl-2,3-dihydro-4H-thiochromeno[4,3-b]furan-4-ones by the action of Zn and ZrCl4 grants access to 4H,5H-thiochromeno[4,3-b]pyran-5-ones.
中文翻译:

2-acyl-2,3-dihydro-4H-thiochromeno[4,3-b]furan-4-ones的三组分合成及其还原重排为4H,5H-thiochromeno[4,3-b]pyran-5 -那些
原位生成的吡啶鎓酰基甲基化物与芳香醛和 4-羟基硫香豆素的三组分缩合导致一系列 2-酰基-2,3-二氢-4 H-硫代色基[4,3 - b ]呋喃-4-酮。该反应非对映选择性地进行,形成反式异构体,代表了一个涉及 Knoevenagel 缩合、碳-迈克尔反应和分子内亲核取代的级联过程。随后通过 Zn 和 ZrCl4 的作用对 2-acyl-2,3-dihydro-4 H -thiochromeno[4,3 - b ]furan-4-ones进行氧化还原重排,获得 4 H ,5 H -thiochromeno[4 ,3 - b ]pyran-5-ones。



























 京公网安备 11010802027423号
京公网安备 11010802027423号