Chemosphere ( IF 8.8 ) Pub Date : 2021-06-05 , DOI: 10.1016/j.chemosphere.2021.131087 Hanyu Wu 1 , Jie Chen 1 , Zengbo Su 2 , Bin Ma 3 , Yizhe Ji 1 , Shuhan Lin 1 , Dingfang Xu 1 , Mingliang Kang 1
|
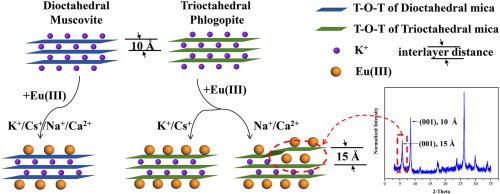
Europium(III), i.e., Eu(III), is chemically analogous to the trivalent lanthanides (Ln) and actinides (An). A good understanding of the adsorption behaviour of Eu(III) on mica group minerals is critical to the safety evaluation of the radioactive contamination. Nevertheless, the structural complexity of micaceous minerals makes it difficult to draw a consistent conclusion in the study of Eu(III) migration. In this work, we contrastively studied Eu(III) adsorption on dioctahedral muscovite and trioctahedral phlogopite as functions of pH, ionic strength, background electrolytes, interaction sequence, and fulvic acid (FA). Batch experiments showed that Eu(III) adsorption on both micas was strongly dependent on pH but quite independent on ionic strength that is determined by Na+. Planar sites are available on both muscovite and phlogopite while interlayer sites only on phlogopite under Na+ and Ca2+ electrolytes (not for K+ and Cs+). An interlayer expansion of phlogopite, as indicated by a newly appeared diffraction peak at ~6° 2-theta, occurred along with Eu(III) adsorption, which was also confirmed by transmission electron microscopy. Furthermore, the initial Eu(III) concentrations, the concentration ratios between Eu(III) and Cs+, and the reaction sequences of Eu(III)-electrolytes-FA affected both the adsorption behaviour of Eu(III) and reversely the structural alteration of phlogopite. The sequential extraction showed that the adsorbed Eu(III) was mainly in the ion-exchangeable form while the addition of FA could increase the portion of coordinative species. The currently proposed Eu(III) adsorption mechanism can shed new light on predicting the migration of Ln/An(III) at the mica-rich solid-liquid interface on a molecular scale.
中文翻译:

深入了解铕 (III) 在白云母和金云母上的吸附:pH 值、电解质、腐殖质和云母结构的影响
铕 (III),即 Eu(III),在化学上类似于三价镧系元素 (Ln) 和锕系元素 (An)。充分了解 Eu(III) 在云母族矿物上的吸附行为对于放射性污染的安全评估至关重要。然而,云母矿物结构的复杂性使得在 Eu(III) 迁移研究中难以得出一致的结论。在这项工作中,我们对比研究了 Eu(III) 在二八面体白云母和三八面体金云母上的吸附,作为 pH、离子强度、背景电解质、相互作用序列和富里酸 (FA) 的函数。批量实验表明,两种云母上的 Eu(III) 吸附强烈依赖于 pH,但完全独立于由 Na +决定的离子强度. 在白云母和金云母上都可以使用平面位点,而在 Na +和 Ca 2+电解质(不适用于 K +和 Cs +)下,仅在金云母上有层间位点。金云母的层间膨胀,如~6° 2-θ 处新出现的衍射峰所示,伴随 Eu(III) 吸附发生,这也通过透射电子显微镜证实。此外,初始 Eu(III) 浓度、Eu(III) 和 Cs +之间的浓度比,Eu(III)-电解质-FA的反应顺序影响Eu(III)的吸附行为和金云母的结构改变。连续萃取表明吸附的Eu(III)主要以离子交换形式存在,而FA的加入可以增加配位物种的比例。目前提出的 Eu(III) 吸附机制可以为预测分子尺度上富含云母的固液界面处的 Ln/An(III) 迁移提供新的思路。


























 京公网安备 11010802027423号
京公网安备 11010802027423号