当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of Poly(glycidyl ether) Structure and Ether Oxygen Placement on CO2 Solubility
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-06-02 , DOI: 10.1021/acs.jced.1c00219 Caitlin L. Bentley 1 , Tangqiumei Song 1 , Benjamin J. Pedretti 1 , Michael J. Lubben 1 , Nathaniel A. Lynd 1 , Joan F. Brennecke 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-06-02 , DOI: 10.1021/acs.jced.1c00219 Caitlin L. Bentley 1 , Tangqiumei Song 1 , Benjamin J. Pedretti 1 , Michael J. Lubben 1 , Nathaniel A. Lynd 1 , Joan F. Brennecke 1
Affiliation
|
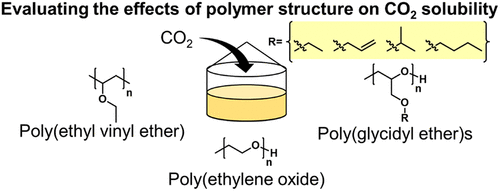
The solubility of CO2 in poly(ethylene oxide), poly(ethyl glycidyl ether), poly(iso-propyl glycidyl ether), poly(allyl glycidyl ether), poly(n-butyl glycidyl ether), and poly(ethyl vinyl ether) was measured at room temperature and 333.15 K and pressures up to 15 bar. CO2 solubility, expressed as mole fraction in terms of molecular weight of the polymer repeat unit, was directly related to polymer repeat unit molecular weight regardless of pendant chain structure or ether oxygen placement in the backbone. The molality of CO2 was highest in poly(ethyl vinyl ether) and was equal in all the poly(glycidyl ethers) and poly(ethylene oxide). The standard enthalpies and entropies of CO2 absorption in poly(ethylene oxide), poly(ethyl glycidyl ether), and poly(ethyl vinyl ether) were calculated from the Henry’s constants obtained from three isotherms. CO2 dissolution was slightly more favorable enthalpically in poly(ethylene oxide). However, the entropic penalty for absorption was lower in poly(ethyl glycidyl ether) and poly(ethyl vinyl ether). These results suggest that poly(glycidyl ethers) and poly(ethyl vinyl ether) are promising alternatives to poly(ethylene oxide) for CO2 separation by absorption or membrane separation because they have similar CO2 uptake capacity, low glass transition temperatures, are amorphous, and are more hydrophobic than poly(ethylene oxide).
中文翻译:

聚(缩水甘油醚)结构和醚氧位置对CO 2溶解度的影响
CO的溶解度2在聚(环氧乙烷),聚(乙基缩水甘油基醚),聚(异-丙基缩水甘油基醚),聚(烯丙基缩水甘油醚),聚(Ñ丁基缩水甘油基醚),和聚(乙基乙烯基醚) 是在室温和 333.15 K 以及高达 15 bar 的压力下测量的。CO 2溶解度,以聚合物重复单元的分子量的摩尔分数表示,与聚合物重复单元的分子量直接相关,而与主链中的侧链结构或醚氧位置无关。CO 2的摩尔浓度在聚(乙基乙烯基醚)中最高,并且在所有聚(缩水甘油醚)和聚(环氧乙烷)中相等。CO 2的标准焓和熵从三个等温线获得的亨利常数计算在聚(环氧乙烷)、聚(乙基缩水甘油醚)和聚(乙基乙烯基醚)中的吸收。CO 2溶解在聚(环氧乙烷)中的焓稍微更有利。然而,聚(乙基缩水甘油醚)和聚(乙基乙烯基醚)的吸收熵损失较低。这些结果表明,聚(缩水甘油醚)和聚(乙基乙烯基醚)是聚(环氧乙烷)的有前途的替代品,用于通过吸收或膜分离进行 CO 2分离,因为它们具有相似的 CO 2吸收能力、低玻璃化转变温度、无定形,并且比聚(环氧乙烷)更疏水。
更新日期:2021-07-08
中文翻译:

聚(缩水甘油醚)结构和醚氧位置对CO 2溶解度的影响
CO的溶解度2在聚(环氧乙烷),聚(乙基缩水甘油基醚),聚(异-丙基缩水甘油基醚),聚(烯丙基缩水甘油醚),聚(Ñ丁基缩水甘油基醚),和聚(乙基乙烯基醚) 是在室温和 333.15 K 以及高达 15 bar 的压力下测量的。CO 2溶解度,以聚合物重复单元的分子量的摩尔分数表示,与聚合物重复单元的分子量直接相关,而与主链中的侧链结构或醚氧位置无关。CO 2的摩尔浓度在聚(乙基乙烯基醚)中最高,并且在所有聚(缩水甘油醚)和聚(环氧乙烷)中相等。CO 2的标准焓和熵从三个等温线获得的亨利常数计算在聚(环氧乙烷)、聚(乙基缩水甘油醚)和聚(乙基乙烯基醚)中的吸收。CO 2溶解在聚(环氧乙烷)中的焓稍微更有利。然而,聚(乙基缩水甘油醚)和聚(乙基乙烯基醚)的吸收熵损失较低。这些结果表明,聚(缩水甘油醚)和聚(乙基乙烯基醚)是聚(环氧乙烷)的有前途的替代品,用于通过吸收或膜分离进行 CO 2分离,因为它们具有相似的 CO 2吸收能力、低玻璃化转变温度、无定形,并且比聚(环氧乙烷)更疏水。



























 京公网安备 11010802027423号
京公网安备 11010802027423号