当前位置:
X-MOL 学术
›
ACS Food Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Comparative Assessment of Thermal Aggregation of Whey, Potato, and Pea Protein under Shear Stress for Microparticulation
ACS Food Science & Technology Pub Date : 2021-06-02 , DOI: 10.1021/acsfoodscitech.1c00104 Caren Tanger 1 , Paola Quintana Ramos 1 , Ulrich Kulozik 1
ACS Food Science & Technology Pub Date : 2021-06-02 , DOI: 10.1021/acsfoodscitech.1c00104 Caren Tanger 1 , Paola Quintana Ramos 1 , Ulrich Kulozik 1
Affiliation
|
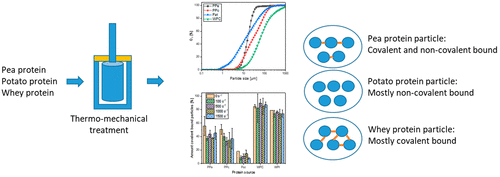
The presented work investigates the aggregation behavior of whey, pea, and potato proteins under a shear stress using a rotational rheometer. The size, protein interaction, and morphology of the aggregates were analyzed. Whey protein particles were cross-linked by disulfide bonds (75%–90%). In contrast, potato protein particles were cross-linked by a hydrophobic interaction (88%–97%). High shear rates were needed to limit the aggregate growth. Pea protein particles were stabilized in equal parts by hydrophobic interactions (40%–62%) and disulfide bonds (37%–56%) in equal parts. Aggregate size was dependent on the processing history of the protein. Native pea protein favored a particle size of 5–30 μm and was independent of the shear rate. An increasing shear rate decreased the aggregate size of preaggregated pea protein to a d50 of 29 μm. A general prediction of the protein aggregation behavior based on the molecular structure remains a challenging task. This research provides insights into the aggregation behavior of pea and potato proteins and helps to design microarticulated structures of plant proteins.
中文翻译:

乳清、马铃薯和豌豆蛋白在剪切应力下微颗粒热聚集的比较评估
目前的工作使用旋转流变仪研究了在剪切应力下乳清、豌豆和马铃薯蛋白质的聚集行为。分析了聚集体的大小、蛋白质相互作用和形态。乳清蛋白颗粒通过二硫键 (75%–90%) 交联。相比之下,马铃薯蛋白颗粒通过疏水相互作用(88%–97%)交联。需要高剪切速率来限制总增长。豌豆蛋白颗粒通过等份的疏水相互作用 (40%–62%) 和二硫键 (37%–56%) 稳定。聚集体大小取决于蛋白质的加工历史。天然豌豆蛋白有利于 5-30 μm 的粒径,并且与剪切速率无关。增加的剪切速率使预先聚集的豌豆蛋白的聚集体尺寸减小到d 50 of 29 μm。基于分子结构的蛋白质聚集行为的一般预测仍然是一项具有挑战性的任务。这项研究提供了对豌豆和马铃薯蛋白质聚集行为的见解,并有助于设计植物蛋白质的微关节结构。
更新日期:2021-06-18
中文翻译:

乳清、马铃薯和豌豆蛋白在剪切应力下微颗粒热聚集的比较评估
目前的工作使用旋转流变仪研究了在剪切应力下乳清、豌豆和马铃薯蛋白质的聚集行为。分析了聚集体的大小、蛋白质相互作用和形态。乳清蛋白颗粒通过二硫键 (75%–90%) 交联。相比之下,马铃薯蛋白颗粒通过疏水相互作用(88%–97%)交联。需要高剪切速率来限制总增长。豌豆蛋白颗粒通过等份的疏水相互作用 (40%–62%) 和二硫键 (37%–56%) 稳定。聚集体大小取决于蛋白质的加工历史。天然豌豆蛋白有利于 5-30 μm 的粒径,并且与剪切速率无关。增加的剪切速率使预先聚集的豌豆蛋白的聚集体尺寸减小到d 50 of 29 μm。基于分子结构的蛋白质聚集行为的一般预测仍然是一项具有挑战性的任务。这项研究提供了对豌豆和马铃薯蛋白质聚集行为的见解,并有助于设计植物蛋白质的微关节结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号