当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanisms of Diels-Alder reactions between pyridines and dienophiles: A DFT investigation
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-06-01 , DOI: 10.1002/poc.4254 Pan‐Pan Zhou 1, 2 , Da‐Gang Zhou 2
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-06-01 , DOI: 10.1002/poc.4254 Pan‐Pan Zhou 1, 2 , Da‐Gang Zhou 2
Affiliation
|
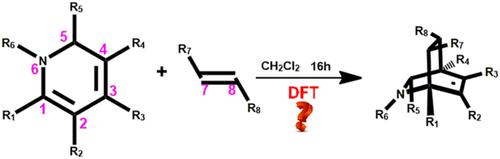
Mechanisms of the Diels-Alder (D-A) reactions between N-alkyl and aryl-1,2-dihydropyridines and dienophiles have been investigated by M06-2X-D3/6-31 + G(d,p) basis set, and SMD model was employed to simulate the solvent effect. The computational results show that the productive process of endo-product is favorable with lower energy barrier. The calculations of substituent effect indicate that there are some relations between the energy barrier and the productivity. The lower energy barrier means the higher productivity, and the barrier of retro D-A reaction could also have influence on the productivity. Finally, natural bond orbital Fukui function (NOFF) and frontier molecular orbital theory (FMOT) are employed to analyze the structures and reveal the substances of D-A reactions.
中文翻译:

吡啶和亲二烯体之间的 Diels-Alder 反应机理:DFT 研究
通过 M06-2X-D3/6-31 + G(d,p) 基组和 SMD 模型研究了N-烷基和芳基-1,2-二氢吡啶与亲二烯体之间的 Diels-Alder (DA) 反应机理用于模拟溶剂效应。计算结果表明,内产物的生产过程是有利的,能量势垒较低。取代基效应的计算表明,能量势垒与生产率之间存在一定的关系。较低的能垒意味着较高的生产率,逆向 DA 反应的势垒也会对生产率产生影响。最后,利用自然键轨道福井函数(NOFF)和前沿分子轨道理论(FMOT)分析了DA反应的结构并揭示了物质。
更新日期:2021-06-01
中文翻译:

吡啶和亲二烯体之间的 Diels-Alder 反应机理:DFT 研究
通过 M06-2X-D3/6-31 + G(d,p) 基组和 SMD 模型研究了N-烷基和芳基-1,2-二氢吡啶与亲二烯体之间的 Diels-Alder (DA) 反应机理用于模拟溶剂效应。计算结果表明,内产物的生产过程是有利的,能量势垒较低。取代基效应的计算表明,能量势垒与生产率之间存在一定的关系。较低的能垒意味着较高的生产率,逆向 DA 反应的势垒也会对生产率产生影响。最后,利用自然键轨道福井函数(NOFF)和前沿分子轨道理论(FMOT)分析了DA反应的结构并揭示了物质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号