当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New Mechanistic Pathways for the Reactions of Formaldehyde with Formic Acid Catalyzed by Sulfuric Acid and Formaldehyde with Sulfuric Acid Catalyzed by Formic Acid: Formation of Potential Secondary Organic Aerosol Precursors
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-06-02 , DOI: 10.1021/acsearthspacechem.1c00002 Ji-Yu Liu 1 , Zheng-Wen Long 1 , Ellen Mitchell 2 , Bo Long 1, 3
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-06-02 , DOI: 10.1021/acsearthspacechem.1c00002 Ji-Yu Liu 1 , Zheng-Wen Long 1 , Ellen Mitchell 2 , Bo Long 1, 3
Affiliation
|
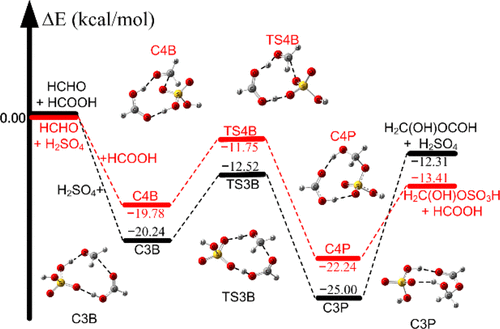
Formaldehyde (HCHO) plays an important role in the formation of secondary organic aerosols. Here, we report new mechanistic pathways for the reactions of formaldehyde with formic acid catalyzed by sulfuric acid and formaldehyde with sulfuric acid catalyzed by formic acid by using quantum chemical methods and reaction rate theory. We have found that sulfuric acid and formic acid have a strong catalytic role in the HCHO···HCOOH + H2SO4 and HCHO···H2SO4 + HCOOH reactions because the calculated energy barriers of the sulfuric acid-catalyzed reaction of formaldehyde with formic acid and formic acid-catalyzed reaction of formaldehyde with sulfuric acid are decreased by 21.17 and 14.81 kcal/mol, respectively. The calculated rates show that the reaction rate of HCHO···HCOOH + H2SO4 is faster than those of HCHO···H2SO4 + HCOOH and HCHO + HCOOH···H2SO4. Moreover, the HCHO + HCOOH + H2SO4 → H2C(OH)OCOH + H2SO4 reaction readily forms the carboxylic acid ester (H2C(OH)OCOH); this is a new mechanistic pathway for the reaction of formaldehyde with formic acid, which is expected to extend to other aldehydes’ reaction with carboxylic acids. Additionally, the present findings also suggest that formic acid-catalyzed formaldehyde and sulfuric acid reaction leads to the formation of an organosulfate as the nucleation precursor. The present results not only help understand the mechanisms for the initial processes of atmospheric nucleation but also are expected to extend other aldehydes and acids in the atmosphere.
中文翻译:

甲酸催化甲醛与甲酸反应以及甲酸催化甲醛与硫酸反应的新机理途径:潜在二次有机气溶胶前体的形成
甲醛 (HCHO) 在二次有机气溶胶的形成中起重要作用。在这里,我们使用量子化学方法和反应速率理论报告了硫酸催化甲醛与甲酸反应以及甲酸催化甲醛与硫酸反应的新机理途径。我们发现硫酸和甲酸对HCHO…HCOOH + H 2 SO 4和HCHO…H 2 SO 4有很强的催化作用+ HCOOH 反应是因为硫酸催化甲醛与甲酸反应和甲酸催化甲醛与硫酸反应的计算能垒分别降低了 21.17 和 14.81 kcal/mol。计算的速率表明,HCHO...HCOOH+H 2 SO 4的反应速度比HCHO...H 2 SO 4 + HCOOH和HCHO+HCOOH...H 2 SO 4的反应速度快。此外,HCHO + HCOOH + H 2 SO 4 → H 2 C(OH)OCOH + H 2 SO 4反应很容易形成羧酸酯(H 2C(OH)OCOH); 这是一种新的甲醛与甲酸反应的机理途径,有望扩展到其他醛与羧酸的反应。此外,目前的发现还表明,甲酸催化的甲醛和硫酸反应导致形成有机硫酸盐作为成核前体。目前的结果不仅有助于理解大气成核初始过程的机制,而且有望扩展大气中的其他醛和酸。
更新日期:2021-06-17
中文翻译:

甲酸催化甲醛与甲酸反应以及甲酸催化甲醛与硫酸反应的新机理途径:潜在二次有机气溶胶前体的形成
甲醛 (HCHO) 在二次有机气溶胶的形成中起重要作用。在这里,我们使用量子化学方法和反应速率理论报告了硫酸催化甲醛与甲酸反应以及甲酸催化甲醛与硫酸反应的新机理途径。我们发现硫酸和甲酸对HCHO…HCOOH + H 2 SO 4和HCHO…H 2 SO 4有很强的催化作用+ HCOOH 反应是因为硫酸催化甲醛与甲酸反应和甲酸催化甲醛与硫酸反应的计算能垒分别降低了 21.17 和 14.81 kcal/mol。计算的速率表明,HCHO...HCOOH+H 2 SO 4的反应速度比HCHO...H 2 SO 4 + HCOOH和HCHO+HCOOH...H 2 SO 4的反应速度快。此外,HCHO + HCOOH + H 2 SO 4 → H 2 C(OH)OCOH + H 2 SO 4反应很容易形成羧酸酯(H 2C(OH)OCOH); 这是一种新的甲醛与甲酸反应的机理途径,有望扩展到其他醛与羧酸的反应。此外,目前的发现还表明,甲酸催化的甲醛和硫酸反应导致形成有机硫酸盐作为成核前体。目前的结果不仅有助于理解大气成核初始过程的机制,而且有望扩展大气中的其他醛和酸。


























 京公网安备 11010802027423号
京公网安备 11010802027423号