当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A density functional theory study of the hydride shift in the Eschweiler–Clarke reaction
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-05-31 , DOI: 10.1002/poc.4253 Shinichi Yamabe 1 , Noriko Tsuchida 2 , Shoko Yamazaki 1
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-05-31 , DOI: 10.1002/poc.4253 Shinichi Yamabe 1 , Noriko Tsuchida 2 , Shoko Yamazaki 1
Affiliation
|
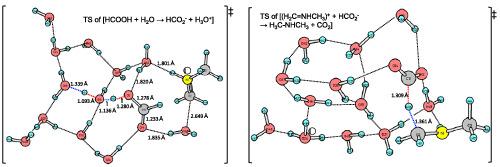
The Eschweiler–Clarke (the amine methylation) reaction was investigated by density functional theory (DFT) calculations. First, a reaction model of H3CNH2 + HC(O)OH + CH2O + (H2O)3 → (H3C)2NH + CO2 + (H2O)4 was employed for geometry optimizations. Geometries and activation free energies of transition states (TSs) by eight DFTs, B2PLYP-D3, B3LYP, B3LYP-D, BP86-D, PBE0-D, M06-2X, wB97X-D, and APF-D, along with MP2 were compared. Four elementary processes were obtained. The rate-determining step is of the hydride-transfer TS, H3CN+HCH2 + HCO2− → H3CNHCH3 + CO2. Whereas BP86-D and APF-D gave underestimated energies, M06-2X was found to be a reasonable DFT to trace the present reaction. Second, by the use of M06-2X/6-311++G**, hydride-shift TSs were examined for various sizes of the water cluster (H2O)n, and the controversial two mechanisms were evaluated. It was suggested that the first formic acid contributes to formation of the iminium ion H3CN+HCH2 and the second formic acid works for the hydride transfer. The Eschweiler–Clarke reaction was described by the scheme, R1R2NH + H2CO + (HCOOH)2 + (H2O)n → R1R2NCH3 + CO2 + HCO2− + H3O+(H2O)n.
中文翻译:

Eschweiler-Clarke反应中氢化物位移的密度泛函理论研究
通过密度泛函理论 (DFT) 计算研究了 Eschweiler-Clarke(胺甲基化)反应。首先,H 3 C NH 2 + HC(O)OH + CH 2 O + (H 2 O) 3 → (H 3 C) 2 NH + CO 2 + (H 2 O) 4 的反应模型是用于几何优化。8 个 DFT、B2PLYP-D3、B3LYP、B3LYP-D、BP86-D、PBE0-D、M06-2X、wB97X-D 和 APF-D 以及 MP2 的几何形状和过渡态 (TS) 的激活自由能是比较的。获得了四个基本过程。速率决定步骤是氢化物转移 TS, H 3 C N + HCH 2 + HCO 2 − → H 3 C NH CH 3 + CO 2。尽管 BP86-D 和 APF-D 给出了低估的能量,但发现 M06-2X 是一个合理的 DFT 来追踪当前的反应。其次,通过使用 M06-2X/6-311++G**,针对不同大小的水团簇 (H 2 O) n检测氢化物位移 TS ,并对有争议的两种机制进行评估。有人提出第一个甲酸有助于形成亚胺离子 H 3 C N + HCH 2第二种甲酸用于氢化物转移。Eschweiler-Clarke 反应由以下方案描述,R 1 R 2 NH + H 2 CO + (HCOOH) 2 + (H 2 O) n → R 1 R 2 NCH 3 + CO 2 + HCO 2 − + H 3 O + (H 2 O) n。
更新日期:2021-05-31
中文翻译:

Eschweiler-Clarke反应中氢化物位移的密度泛函理论研究
通过密度泛函理论 (DFT) 计算研究了 Eschweiler-Clarke(胺甲基化)反应。首先,H 3 C NH 2 + HC(O)OH + CH 2 O + (H 2 O) 3 → (H 3 C) 2 NH + CO 2 + (H 2 O) 4 的反应模型是用于几何优化。8 个 DFT、B2PLYP-D3、B3LYP、B3LYP-D、BP86-D、PBE0-D、M06-2X、wB97X-D 和 APF-D 以及 MP2 的几何形状和过渡态 (TS) 的激活自由能是比较的。获得了四个基本过程。速率决定步骤是氢化物转移 TS, H 3 C N + HCH 2 + HCO 2 − → H 3 C NH CH 3 + CO 2。尽管 BP86-D 和 APF-D 给出了低估的能量,但发现 M06-2X 是一个合理的 DFT 来追踪当前的反应。其次,通过使用 M06-2X/6-311++G**,针对不同大小的水团簇 (H 2 O) n检测氢化物位移 TS ,并对有争议的两种机制进行评估。有人提出第一个甲酸有助于形成亚胺离子 H 3 C N + HCH 2第二种甲酸用于氢化物转移。Eschweiler-Clarke 反应由以下方案描述,R 1 R 2 NH + H 2 CO + (HCOOH) 2 + (H 2 O) n → R 1 R 2 NCH 3 + CO 2 + HCO 2 − + H 3 O + (H 2 O) n。


























 京公网安备 11010802027423号
京公网安备 11010802027423号