当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
MED12 interacts with the heat-shock transcription factor HSF1 and recruits CDK8 to promote the heat-shock response in mammalian cells
FEBS Letters ( IF 3.5 ) Pub Date : 2021-05-31 , DOI: 10.1002/1873-3468.14139 Pratibha Srivastava 1 , Ryosuke Takii 1 , Mariko Okada 1 , Mitsuaki Fujimoto 1 , Akira Nakai 1
FEBS Letters ( IF 3.5 ) Pub Date : 2021-05-31 , DOI: 10.1002/1873-3468.14139 Pratibha Srivastava 1 , Ryosuke Takii 1 , Mariko Okada 1 , Mitsuaki Fujimoto 1 , Akira Nakai 1
Affiliation
|
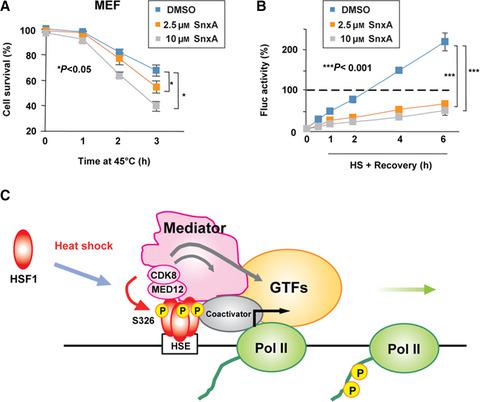
Activated and promoter-bound heat-shock transcription factor 1 (HSF1) induces RNA polymerase II recruitment upon heat shock, and this is facilitated by the core Mediator in Drosophila and yeast. Another Mediator module, CDK8 kinase module (CKM), consisting of four subunits including MED12 and CDK8, plays a negative or positive role in the regulation of transcription; however, its involvement in HSF1-mediated transcription remains unclear. We herein demonstrated that HSF1 interacted with MED12 and recruited MED12 and CDK8 to the HSP70 promoter during heat shock in mammalian cells. The kinase activity of CDK8 (and its paralog CDK19) promoted HSP70 expression partly by phosphorylating HSF1-S326 and maintained proteostasis capacity. These results indicate an important role for CKM in the protection of cells against proteotoxic stress.
中文翻译:

MED12 与热休克转录因子 HSF1 相互作用并募集 CDK8 以促进哺乳动物细胞的热休克反应
激活的和启动子结合的热休克转录因子 1 (HSF1) 在热休克时诱导 RNA 聚合酶 II 募集,这是由果蝇和酵母中的核心介质促进的。另一个Mediator模块,CDK8激酶模块(CKM),由MED12和CDK8等4个亚基组成,在转录调控中起正负作用;然而,其参与 HSF1 介导的转录仍不清楚。我们在此证明了 HSF1 与 MED12 相互作用并将 MED12 和 CDK8在哺乳动物细胞的热休克过程中募集到HSP70启动子。CDK8(及其旁系同源物 CDK19)的激酶活性促进了HSP70部分通过磷酸化 HSF1-S326 并保持蛋白质稳态能力来表达。这些结果表明 CKM 在保护细胞免受蛋白毒性应激方面的重要作用。
更新日期:2021-07-27
中文翻译:

MED12 与热休克转录因子 HSF1 相互作用并募集 CDK8 以促进哺乳动物细胞的热休克反应
激活的和启动子结合的热休克转录因子 1 (HSF1) 在热休克时诱导 RNA 聚合酶 II 募集,这是由果蝇和酵母中的核心介质促进的。另一个Mediator模块,CDK8激酶模块(CKM),由MED12和CDK8等4个亚基组成,在转录调控中起正负作用;然而,其参与 HSF1 介导的转录仍不清楚。我们在此证明了 HSF1 与 MED12 相互作用并将 MED12 和 CDK8在哺乳动物细胞的热休克过程中募集到HSP70启动子。CDK8(及其旁系同源物 CDK19)的激酶活性促进了HSP70部分通过磷酸化 HSF1-S326 并保持蛋白质稳态能力来表达。这些结果表明 CKM 在保护细胞免受蛋白毒性应激方面的重要作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号