Water Research ( IF 12.8 ) Pub Date : 2021-05-31 , DOI: 10.1016/j.watres.2021.117314 Zhiruo Zhou 1 , Zhurui Shen 2 , Chunlin Song 1 , Mingmei Li 1 , Hui Li 3 , Sihui Zhan 4
|
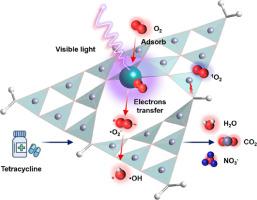
Photocatalytic activation of molecular oxygen (O2) is a promising way in oxidative degradation of organic pollutants. However, it suffers from low efficiency mainly due to the limited active sites for O2 activation over traditional photocatalysts. Therefore, we established a single atomic Ag-g-C3N4 (SAACN) catalyst with 10 wt% loading of Ag single sites for boosting the O2 activation during the degradation of tetracycline (TC), and 10 wt% loading of nanoparticle Ag-g-C3N4 (NPACN) was studied as a comparison. When using SAACN, the accumulative concentration of superoxide (•O2−), hydroxyl radical (•OH), singlet oxygen (1O2) reached up to 0.66, 0.19, 0.33 mmol L−1h−1, respectively, within 120 min, 11.7, 5.7 and 4.9 times compared with those using NPACN, representing 17.24% of dissolved O2 was converted to reactive oxygen species (ROS). When additionally feeding air or O2, the accumulative concentrations of •O2−, •OH, 1O2 were even higher (air: 4.21, 0.97, 2.02 mmol L−1 h−1; O2: 17.13, 1.32, 9.00 mmol L−1 h−1). The rate constants (k) for degrading the TC were 0.0409 min−1 over SAACN and 0.00880 min−1 over NPACN, respectively (mineralization rate: 95.7% vs. 59.9% after 3 h of degradation). Moreover, the degradation ability of SAACN did not decrease in a wide range of pH value (4–10) or under low temperature (10 °C). Besides the high exposure of Ag single sites, other advances of SAACN were: 1(O2 was more energetic favorable to adsorb on single atomic Ag sites; 2) Positive Ag single sites were easier to obtain the electrons from the surrounding N atoms, and facilitated electron transfer towards adsorbed O2.
中文翻译:

在高负载Ag单原子催化剂上促进分子氧的活化和四环素的降解
分子氧(O 2)的光催化活化是氧化降解有机污染物的一种很有前景的方法。然而,它的效率低下主要是由于与传统光催化剂相比,O 2活化的活性位点有限。因此,我们建立了一种单原子Ag- g -C 3 N 4 (SAACN) 催化剂,其负载量为 10 wt% 的 Ag 单点,用于在四环素 (TC) 降解过程中促进 O 2活化,以及负载量为 10 wt% 的纳米颗粒AG-克-C 3 ñ 4(NPACN)进行了研究作为比较。使用 SAACN 时,超氧化物 (•O 2 -)、羟基自由基 (•OH)、单线态氧 ( 1 O 2 )在 120 分钟内分别达到 0.66、0.19、0.33 mmol L -1 h -1,是使用 NPACN 的 11.7、5.7 和 4.9 倍,代表 17.24% 的溶解 O 2被转化为活性氧 (ROS)。当额外加入空气或O 2 时, •O 2 -、•OH、1 O 2的累积浓度甚至更高(空气:4.21、0.97、2.02 mmol L -1 h -1;O 2:17.13、1.32、9.00 mmol L -1 h -1 )。速率常数 (ķ)降解的TC为0.0409分钟-1以上SAACN和0.00880分钟-1以上NPACN,分别为(矿化速率:95.7%比。后59.9%的降解的3小时)。此外,SAACN 的降解能力在较宽的 pH 值范围(4-10)或低温(10°C)下没有降低。除了Ag单点的高暴露外,SAACN的其他优点是:1(O 2更有利于吸附在单原子Ag位点上;2)正的Ag单点更容易从周围的N原子中获得电子,和促进电子向吸附的 O 2转移。



























 京公网安备 11010802027423号
京公网安备 11010802027423号