Chemical & Pharmaceutical Bulletin ( IF 1.7 ) Pub Date : 2021-06-01 , DOI: 10.1248/cpb.c21-00047 Takashi Ishizu 1 , Miku Tokunaga 1 , Moeka Fukuda 1 , Mana Matsumoto 1 , Takeshi Goromaru 1 , Soushi Takemoto 1
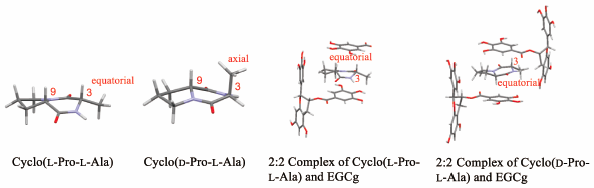
The addition of an aqueous solution of diketopiperazine cyclo(Pro-Xxx) (Xxx: amino acid residue) to an aqueous solution of (−)-epigallocatechin-3-O-gallate (EGCg) led to precipitation of the complex of EGCg and cyclo(Pro-Xxx). The molecular capture abilities of cyclo(Pro-Xxx) using EGCg were evaluated by the ratio of the amount of cyclo(Pro-Xxx) included in the precipitates of the complex with EGCg to that of the total cyclo(Pro-Xxx) used. Stronger hydrophobicity of the side chain of the amino acid residue of cyclo(Pro-Xxx) led to a higher molecular capture ability. Furthermore, the molecular capture ability decreased when the side chain of the amino acid residue had a hydrophilic hydroxyl group. When diketopiperazine cyclo(Pro-Xxx), excluding cyclo(D-Pro-L-Ala), was taken into the hydrophobic space formed by the three aromatic A, B, and B′ rings of EGCg, and formed a complex, their conformation was maintained in the hydrophobic space. Based on nuclear Overhauser effect (NOE) measurement, the 3-position methyl group of cyclo(D-Pro-L-Ala) in D2O was axial, whereas that of cyclo(L-Pro-L-Ala) was equatorial. When cyclo(D-Pro-L-Ala) was taken into the hydrophobic space of EGCg and formed a 2 : 2 complex, its 3-position methyl group changed from the axial position to the equatorial position due to steric hindrance by EGCg.
 Fullsize Image
Fullsize Image
中文翻译:

表没食子儿茶素-3-O-没食子酸酯在水中对含有脯氨酸残基的二酮哌嗪的分子捕获和构象变化
将二酮哌嗪环(Pro-Xxx)(Xxx:氨基酸残基)的水溶液添加到(-)-表没食子儿茶素-3- O的水溶液中-gallate (EGCg) 导致 EGCg 和环 (Pro-Xxx) 的复合物沉淀。使用 EGCg 的环 (Pro-Xxx) 的分子捕获能力通过与 EGCg 复合物的沉淀物中包含的环 (Pro-Xxx) 的量与所用的总环 (Pro-Xxx) 的量之比来评估。cyclo(Pro-Xxx) 氨基酸残基侧链的更强疏水性导致更高的分子捕获能力。此外,当氨基酸残基的侧链具有亲水性羟基时,分子捕获能力降低。当不包括环(D-Pro-L-Ala)的二酮哌嗪环(Pro-Xxx)被带入EGCg的三个芳香A、B和B'环形成的疏水空间中,形成复合物时,它们的构象保持在疏水空间中。基于核奥弗豪泽效应 (NOE) 测量,2 O 是轴向的,而环(L-Pro-L-Ala)是赤道的。当环(D-Pro-L-Ala)进入EGCg的疏水空间并形成2:2复合物时,由于EGCg的空间位阻,其3位甲基从轴向位置变为赤道位置。
 全尺寸图像
全尺寸图像



























 京公网安备 11010802027423号
京公网安备 11010802027423号