Journal of Physics and Chemistry of Solids ( IF 4 ) Pub Date : 2021-05-30 , DOI: 10.1016/j.jpcs.2021.110177 Harrison Henri dos Santos Nascimento , Marcio Luis Ferreira Nascimento
|
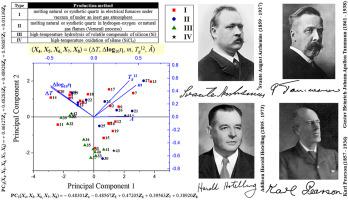
As the most important glass former, silica has a plethora of relevant industrial and commercial applications. Silica glass can be classified into several types depending on the starting materials, production method, content and impurities. Briefly, type I is obtained by melting natural or synthetic quartz in electrical furnaces, presenting alkali and metal impurities (in ppm). Type II is obtained from melting natural or synthetic quartz in hydrogen–oxygen or natural gas flames with few impurities (in ppm) but high concentration of structural water. Type III is obtained from high-temperature hydrolysis of volatile compounds of silicon with low metal impurities and considerable concentrations of structural water and chlorine. Type IV is obtained from high-temperature oxidation of silane with small amounts of metal impurities, almost no structural water but high chlorine content. We analyzed literature data on viscosity, η, for 44 batches over a wide temperature range, from glass transition, Tg, to far beyond the melting point, Tm, for four of five silica types. The viscosity behavior in silica follows the Arrhenius expression: with η in Pa⋅s, T in K, where A and B are constants. However, the Vogel-Fulcher-Tammann-Hesse (VFTH) equation: can also be applied to this system, with T0 → 0 as another constant (in K). For a fixed temperature, a variance of η, covering two orders of magnitude, was observed for types I and II, and about one order for the other two types, mainly due to impurity content and the manufacturing process. Another important observation was that the highest viscosities were found in the purest silicas. Not all samples presented T0 = 0, as expected for Arrhenius viscosity behavior. An analysis considering the Mauro-Yue-Ellison-Gupta-Allan (MYEGA) viscosity equation was also performed. Previous studies of principal component analysis (PCA) considering A, B and T0 allowed the mapping of binary and ternary silicate and borate systems. In brief, PCA is a mathematical technique which rearranges information from datasets and can classify silica types according to their viscosity parameters. The results show that it is possible to map the four silica glass types according to viscosity VFTH and MYEGA parameters taken from experimental data.
中文翻译:

使用粘度数据和主成分分析识别二氧化硅类型
作为最重要的玻璃形成剂,二氧化硅具有大量相关的工业和商业应用。石英玻璃根据原料、生产方法、含量和杂质的不同,可分为几种类型。简而言之,I 型是通过在电炉中熔化天然或合成石英获得的,存在碱和金属杂质(以 ppm 为单位)。II 型是通过在氢-氧或天然气火焰中熔化天然或合成石英而获得的,杂质很少(以 ppm 为单位)但结构水浓度很高。III型是通过高温水解具有低金属杂质和相当浓度的结构水和氯的硅挥发性化合物而获得的。IV型是由含有少量金属杂质的硅烷高温氧化得到的,几乎没有结构水,但氯含量高。我们分析了 44 个批次在很宽的温度范围内的粘度 η 的文献数据,从玻璃化转变、对于五种二氧化硅类型中的四种,T g远远超过熔点T m。二氧化硅中的粘度行为遵循 Arrhenius 表达式:η 在 Pa⋅s 中,T在 K 中,其中A和B是常数。然而,Vogel-Fulcher-Tammann-Hesse (VFTH) 方程:也可以应用于这个系统,T 0 → 0 作为另一个常数(以 K 为单位)。对于固定温度,I 型和 II 型观察到 η 的变化,涵盖两个数量级,其他两种类型的变化约为一个数量级,主要是由于杂质含量和制造工艺。另一个重要的观察结果是在最纯的二氧化硅中发现了最高的粘度。并非所有样品都呈现T 0 = 0,正如 Arrhenius 粘度行为所预期的那样。还进行了考虑 Mauro-Yue-Ellison-Gupta-Allan (MYEGA) 粘度方程的分析。考虑A、B和T 0的主成分分析 (PCA) 的先前研究允许绘制二元和三元硅酸盐和硼酸盐系统。简而言之,PCA 是一种数学技术,它重新排列数据集中的信息,并可以根据其粘度参数对二氧化硅类型进行分类。结果表明,可以根据从实验数据中获取的粘度 VFTH 和 MYEGA 参数来绘制四种石英玻璃类型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号