当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The reported formation of 5H-dibenzo[b,e][1,4]diazepin-11(10H)-ones in the noncatalyzed, base-promoted double arylation of anthranilamide revisited. Correction of some product structures
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2021-05-30 , DOI: 10.1002/jhet.4311 Zbigniew Wróbel 1 , Bogdan Wilk 2 , Piotr Cmoch 1 , Andrzej Kwast 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2021-05-30 , DOI: 10.1002/jhet.4311 Zbigniew Wróbel 1 , Bogdan Wilk 2 , Piotr Cmoch 1 , Andrzej Kwast 1
Affiliation
|
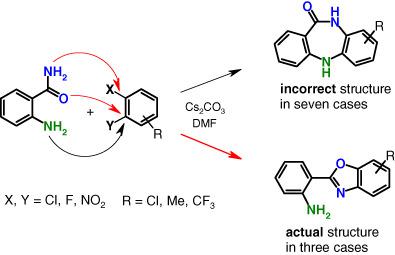
The base-promoted reaction of 2-halonitro- or 1,2-dihalobenzenes with anthranilamide reported by Cao, Ma et al. (Synthesis 2013, 45, 111) was reinvestigated. Some of the products reported, which have been identified as dibenzodiazepinones, are actually benzoxazole derivatives. In this paper, the correct structures of these products were established and confirmed by independent synthesis. For four other products, the supposed structures were found to be incompatible with the dibenzodiazepinones that were synthesized by the reliable method used in this work.
中文翻译:

重新审视了 5H-二苯并[b,e][1,4]diazepin-11(10H)-ones 在非催化、碱促进的邻氨基苯甲酸双芳基化中的形成。修正部分产品结构
Cao、Ma 等人报道的 2-卤代硝基苯或 1,2-二卤代苯与邻氨基苯甲酰胺的碱促进反应。( Synthesis 2013 , 45 , 111) 被重新研究。一些报告的产品已被确定为二苯并二氮杂酮,实际上是苯并恶唑衍生物。在本文中,这些产品的正确结构是通过独立合成建立和确认的。对于其他四种产品,发现假定的结构与通过本工作中使用的可靠方法合成的二苯并二氮杂酮类化合物不相容。
更新日期:2021-05-30
中文翻译:

重新审视了 5H-二苯并[b,e][1,4]diazepin-11(10H)-ones 在非催化、碱促进的邻氨基苯甲酸双芳基化中的形成。修正部分产品结构
Cao、Ma 等人报道的 2-卤代硝基苯或 1,2-二卤代苯与邻氨基苯甲酰胺的碱促进反应。( Synthesis 2013 , 45 , 111) 被重新研究。一些报告的产品已被确定为二苯并二氮杂酮,实际上是苯并恶唑衍生物。在本文中,这些产品的正确结构是通过独立合成建立和确认的。对于其他四种产品,发现假定的结构与通过本工作中使用的可靠方法合成的二苯并二氮杂酮类化合物不相容。


























 京公网安备 11010802027423号
京公网安备 11010802027423号