当前位置:
X-MOL 学术
›
Z. Anorg. Allg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iron(II) Complexes Featuring a Redox-Active Dihydrazonopyrrole Ligand
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2021-05-27 , DOI: 10.1002/zaac.202100097 Kate A. Jesse, Mu-Chieh Chang, Alexander S. Filatov, John S. Anderson
Zeitschrift für anorganische und allgemeine Chemie ( IF 1.4 ) Pub Date : 2021-05-27 , DOI: 10.1002/zaac.202100097 Kate A. Jesse, Mu-Chieh Chang, Alexander S. Filatov, John S. Anderson
|
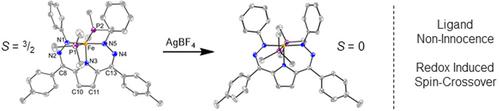
Nature uses control of the secondary coordination sphere to facilitate an astounding variety of transformations. Similarly, synthetic chemists have found metal-ligand cooperativity to be a powerful strategy for designing complexes that can mediate challenging reactivity. In particular, this strategy has been used to facilitate two electron reactions with first row transition metals that more typically engage in one electron redox processes. While NNN pincer ligands feature prominently in this area, examples which can potentially engage in both proton and electron transfer are less common. Dihydrazonopyrrole (DHP) ligands have been isolated in a variety of redox and protonation states when complexed to Ni. However, the redox-state of this ligand scaffold is less obvious when complexed to metal centers with more accessible redox couples. Here, we synthesize a new series of Fe-DHP complexes in two distinct oxidation states. Detailed characterization supports that the redox-chemistry in this set is still primarily ligand based. Finally, these complexes exist as 5-coordinate species with an open coordination site offering the possibility of enhanced reactivity.
中文翻译:

具有氧化还原活性的二肼吡咯配体的铁 (II) 配合物
大自然使用对二级协调领域的控制来促进惊人的变化。同样,合成化学家发现金属-配体协同作用是设计可以调节具有挑战性的反应性的复合物的有力策略。特别是,该策略已被用于促进与第一行过渡金属的两个电子反应,这些过渡金属更典型地参与一个电子氧化还原过程。虽然 NNN 钳形配体在该领域具有突出的特点,但可能同时参与质子和电子转移的例子并不常见。Dihydrazonopyrrole (DHP) 配体在与 Ni 络合时以多种氧化还原和质子化状态分离。然而,当用更容易接近的氧化还原对与金属中心络合时,这种配体支架的氧化还原状态就不那么明显了。这里,我们以两种不同的氧化态合成了一系列新的 Fe-DHP 配合物。详细的表征支持该组中的氧化还原化学仍然主要基于配体。最后,这些配合物以 5 配位物种的形式存在,具有开放的配位点,提供了增强反应性的可能性。
更新日期:2021-07-14
中文翻译:

具有氧化还原活性的二肼吡咯配体的铁 (II) 配合物
大自然使用对二级协调领域的控制来促进惊人的变化。同样,合成化学家发现金属-配体协同作用是设计可以调节具有挑战性的反应性的复合物的有力策略。特别是,该策略已被用于促进与第一行过渡金属的两个电子反应,这些过渡金属更典型地参与一个电子氧化还原过程。虽然 NNN 钳形配体在该领域具有突出的特点,但可能同时参与质子和电子转移的例子并不常见。Dihydrazonopyrrole (DHP) 配体在与 Ni 络合时以多种氧化还原和质子化状态分离。然而,当用更容易接近的氧化还原对与金属中心络合时,这种配体支架的氧化还原状态就不那么明显了。这里,我们以两种不同的氧化态合成了一系列新的 Fe-DHP 配合物。详细的表征支持该组中的氧化还原化学仍然主要基于配体。最后,这些配合物以 5 配位物种的形式存在,具有开放的配位点,提供了增强反应性的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号