当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CO2 utilization in built environment via the PCO2 swing carbonation of alkaline solid wastes with different mineralogy
Faraday Discussions ( IF 3.4 ) Pub Date : 2021-3-12 , DOI: 10.1039/d1fd00022e Guanhe Rim 1 , Noyonika Roy 2 , Diandian Zhao 3 , Shiho Kawashima 3 , Phillip Stallworth 4 , Steven G Greenbaum 4 , Ah-Hyung Alissa Park 1
Faraday Discussions ( IF 3.4 ) Pub Date : 2021-3-12 , DOI: 10.1039/d1fd00022e Guanhe Rim 1 , Noyonika Roy 2 , Diandian Zhao 3 , Shiho Kawashima 3 , Phillip Stallworth 4 , Steven G Greenbaum 4 , Ah-Hyung Alissa Park 1
Affiliation
|
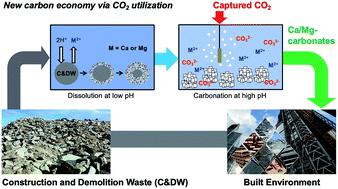
Carbon mineralization to solid carbonates is one of the reaction pathways that can not only utilize captured CO2 but also potentially store it in the long term. In this study, the dissolution and carbonation behaviors of alkaline solid wastes (i.e., waste concrete) was investigated. Concrete is one of the main contributors to a large carbon emission in the built environment. Thus, the upcycling of waste concrete via CO2 utilization has multifaceted environmental benefits including CO2 emission reduction, waste management and reduced mining. Unlike natural silicate minerals such as olivine and serpentine, alkaline solid wastes including waste concrete are highly reactive, and thus, their dissolution and carbonation behaviors vary significantly. Here, both conventional acid (e.g., hydrochloric acid) and less studied carbonic acid (i.e., CO2 saturated water) solvent systems were explored to extract Ca from concrete. Non-stoichiometric dissolution behaviors between Ca and Si were confirmed under far-from-equilibrium conditions (0.1 wt% slurry density), and the re-precipitation of the extracted Si was observed at near-equilibrium conditions (5 wt% slurry density), when the Ca extraction was performed at a controlled pH of 3. These experiments, with a wide range of slurry densities, provided valuable insight into Si re-precipitation phenomena and its effect on the mass transfer limitation during concrete dissolution. Next, the use of the partial pressure of CO2 for the pH swing carbon mineralization process was investigated for concrete, and the results were compared to those of Mg-bearing silicate minerals. In the PCO2 swing process, the extraction of Ca was significantly limited by the precipitation of the carbonate phase (i.e., calcite), since CO2 bubbling could not provide a low enough pH condition for concrete–water–CO2 systems. Thus, this study showed that the two-step carbon mineralization via PCO2 swing, that has been developed for Mg-bearing silicate minerals, may not be viable for highly reactive Ca-bearing silicate materials (e.g., concrete). The precipitated calcium carbonate (PCC) derived from waste concrete via a pH swing process showed very promising results with a high CO2 utilization potential as an upcycled construction material.
中文翻译:

通过不同矿物的碱性固体废物的 PCO2 摆动碳酸化在建筑环境中利用 CO2
碳矿化为固体碳酸盐是反应途径之一,它不仅可以利用捕获的 CO 2还可以长期储存它。在这项研究中,研究了碱性固体废物(即废弃混凝土)的溶解和碳化行为。混凝土是建筑环境中大量碳排放的主要贡献者之一。因此,通过利用CO 2对废弃混凝土进行升级回收具有多方面的环境效益,包括 CO 2减排、废物管理和减少采矿。与橄榄石和蛇纹石等天然硅酸盐矿物不同,废混凝土等碱性固体废物具有高反应性,因此其溶解和碳化行为差异很大。在这里,常规酸(例如盐酸)和研究较少的碳酸(即CO 2饱和水)溶剂系统被探索从混凝土中提取钙。在远离平衡的条件下(0.1 wt% 的浆液密度)证实了 Ca 和 Si 之间的非化学计量溶解行为,并且在接近平衡的条件下(5 wt% 的浆液密度)观察到了提取的 Si 的再沉淀,当在 3 的受控 pH 值下进行 Ca 提取时,这些实验具有广泛的浆料密度,提供了对 Si 再沉淀现象及其对混凝土溶解过程中传质限制的影响的宝贵见解。接下来,研究了 CO 2分压在混凝土 pH 变化碳矿化过程中的使用,并将结果与含镁硅酸盐矿物的结果进行了比较。在P CO在 2摆动过程中,Ca 的提取受到碳酸盐相(即方解石)沉淀的显着限制,因为 CO2鼓泡不能为混凝土-水-CO2系统提供足够低的 pH 条件。因此,这项研究表明,已经为含镁硅酸盐矿物开发的通过 PCO 2摆动的两步碳矿化可能不适用于高活性含钙硅酸盐材料(例如混凝土)。通过pH 波动过程从废弃混凝土中提取的沉淀碳酸钙 (PCC)显示出非常有希望的结果,具有高 CO2 作为升级回收的建筑材料的利用潜力。
更新日期:2021-03-12
中文翻译:

通过不同矿物的碱性固体废物的 PCO2 摆动碳酸化在建筑环境中利用 CO2
碳矿化为固体碳酸盐是反应途径之一,它不仅可以利用捕获的 CO 2还可以长期储存它。在这项研究中,研究了碱性固体废物(即废弃混凝土)的溶解和碳化行为。混凝土是建筑环境中大量碳排放的主要贡献者之一。因此,通过利用CO 2对废弃混凝土进行升级回收具有多方面的环境效益,包括 CO 2减排、废物管理和减少采矿。与橄榄石和蛇纹石等天然硅酸盐矿物不同,废混凝土等碱性固体废物具有高反应性,因此其溶解和碳化行为差异很大。在这里,常规酸(例如盐酸)和研究较少的碳酸(即CO 2饱和水)溶剂系统被探索从混凝土中提取钙。在远离平衡的条件下(0.1 wt% 的浆液密度)证实了 Ca 和 Si 之间的非化学计量溶解行为,并且在接近平衡的条件下(5 wt% 的浆液密度)观察到了提取的 Si 的再沉淀,当在 3 的受控 pH 值下进行 Ca 提取时,这些实验具有广泛的浆料密度,提供了对 Si 再沉淀现象及其对混凝土溶解过程中传质限制的影响的宝贵见解。接下来,研究了 CO 2分压在混凝土 pH 变化碳矿化过程中的使用,并将结果与含镁硅酸盐矿物的结果进行了比较。在P CO在 2摆动过程中,Ca 的提取受到碳酸盐相(即方解石)沉淀的显着限制,因为 CO2鼓泡不能为混凝土-水-CO2系统提供足够低的 pH 条件。因此,这项研究表明,已经为含镁硅酸盐矿物开发的通过 PCO 2摆动的两步碳矿化可能不适用于高活性含钙硅酸盐材料(例如混凝土)。通过pH 波动过程从废弃混凝土中提取的沉淀碳酸钙 (PCC)显示出非常有希望的结果,具有高 CO2 作为升级回收的建筑材料的利用潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号