当前位置:
X-MOL 学术
›
J. Phys. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical kinetic studies of ethynyl radical with n-butane
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-05-24 , DOI: 10.1002/poc.4249 Manas Ranjan Dash 1 , Subhashree Subhadarsini Mishra 2
Journal of Physical Organic Chemistry ( IF 1.8 ) Pub Date : 2021-05-24 , DOI: 10.1002/poc.4249 Manas Ranjan Dash 1 , Subhashree Subhadarsini Mishra 2
Affiliation
|
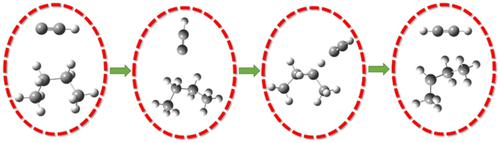
The rate coefficients of ethynyl radical reaction with n-butane were computed for the first time using the canonical variational transition state theory (CVT) between 150 and 5000 K. The structures and frequencies of all the stationary points are computed at the M06-2X/6-31+G(d,p) level of theory. The potential energy surface scanned shows that two rotamers exist for n-butane. Three different transition states were identified for each rotamers. The rate coefficients obtained over the temperature range of 150–500 K using the M06-2X/6-31+G(d,p) theory were used to derive the Arrhenius expression: k(T) = 1.35 × 10−17 T2.0 exp[1326/T] cm3 molecule−1 s−1. It is shown that the H-abstraction from –CH2– group of n-butane is the dominant reaction channel over the complete temperature range.
中文翻译:

乙炔自由基与正丁烷的理论动力学研究
首次使用典型变分过渡态理论 (CVT) 计算了 150 至 5000 K 之间乙炔自由基与正丁烷反应的速率系数。所有驻点的结构和频率均在 M06-2X/ 6-31+G(d,p) 理论水平。扫描的势能表面显示正丁烷存在两个旋转异构体。为每个旋转异构体确定了三种不同的过渡态。使用 M06-2X/6-31+G(d,p) 理论在 150–500 K 温度范围内获得的速率系数用于推导 Arrhenius 表达式:k (T) = 1.35 × 10 -17 T 2.0 exp[1326/T] cm 3 分子-1 s -1. 结果表明,在整个温度范围内,正丁烷的–CH 2 – 基团的H-抽提是主要的反应通道。
更新日期:2021-05-24
中文翻译:

乙炔自由基与正丁烷的理论动力学研究
首次使用典型变分过渡态理论 (CVT) 计算了 150 至 5000 K 之间乙炔自由基与正丁烷反应的速率系数。所有驻点的结构和频率均在 M06-2X/ 6-31+G(d,p) 理论水平。扫描的势能表面显示正丁烷存在两个旋转异构体。为每个旋转异构体确定了三种不同的过渡态。使用 M06-2X/6-31+G(d,p) 理论在 150–500 K 温度范围内获得的速率系数用于推导 Arrhenius 表达式:k (T) = 1.35 × 10 -17 T 2.0 exp[1326/T] cm 3 分子-1 s -1. 结果表明,在整个温度范围内,正丁烷的–CH 2 – 基团的H-抽提是主要的反应通道。



























 京公网安备 11010802027423号
京公网安备 11010802027423号