当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Class A G protein-coupled receptors assemble into functional higher-order hetero-oligomers
FEBS Letters ( IF 3.5 ) Pub Date : 2021-05-25 , DOI: 10.1002/1873-3468.14135 Xianlong Gao 1 , Garrett A Enten 1, 2 , Anthony J DeSantis 1 , Matthias Majetschak 1, 2
FEBS Letters ( IF 3.5 ) Pub Date : 2021-05-25 , DOI: 10.1002/1873-3468.14135 Xianlong Gao 1 , Garrett A Enten 1, 2 , Anthony J DeSantis 1 , Matthias Majetschak 1, 2
Affiliation
|
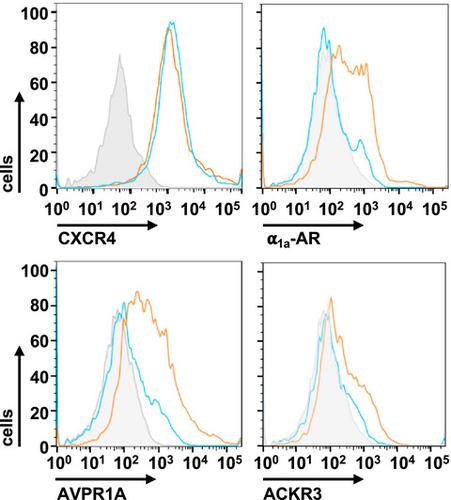
Although class A seven-transmembrane helix (7TM) receptor hetero-oligomers have been proposed, information on the assembly and function of such higher-order hetero-oligomers is not available. Utilizing bioluminescence resonance energy transfer (BRET), bimolecular luminescence/fluorescence complementation (BiLC/BiFC), and BiLC/BiFC BRET in HEK293T cells, we provide evidence that chemokine (C-X-C motif) receptor 4, atypical chemokine receptor 3, α1a-adrenoceptor, and arginine vasopressin receptor 1A form hetero-oligomers composed of 2–4 different protomers. We show that hetero-oligomerization per se and ligand binding to individual protomers regulate agonist-induced coupling to the signaling transducers of interacting receptor partners. Our findings support the concept that receptor hetero-oligomers form supramolecular machineries with molecular signaling properties distinct from the individual protomers. These findings provide a mechanism for the phenomenon of context-dependent receptor function.
中文翻译:

AG 类蛋白偶联受体组装成功能性高阶异源寡聚体
尽管已经提出了 A 类七跨膜螺旋 (7TM) 受体异源寡聚体,但尚无关于此类高阶异源寡聚体的组装和功能的信息。利用 HEK293T 细胞中的生物发光共振能量转移 (BRET)、双分子发光/荧光互补 (BiLC/BiFC) 和 BiLC/BiFC BRET,我们提供了趋化因子 (CXC 基序) 受体 4、非典型趋化因子受体 3、α 1a的证据肾上腺素受体和精氨酸加压素受体 1A 形成由 2-4 个不同的原体组成的异源寡聚体。我们表明,异源寡聚本身和与单个原体结合的配体调节激动剂诱导的与相互作用受体伙伴的信号转导器的耦合。我们的研究结果支持受体异源寡聚体形成具有不同于单个原体的分子信号传导特性的超分子机器的概念。这些发现为上下文依赖性受体功能的现象提供了一种机制。
更新日期:2021-07-27
中文翻译:

AG 类蛋白偶联受体组装成功能性高阶异源寡聚体
尽管已经提出了 A 类七跨膜螺旋 (7TM) 受体异源寡聚体,但尚无关于此类高阶异源寡聚体的组装和功能的信息。利用 HEK293T 细胞中的生物发光共振能量转移 (BRET)、双分子发光/荧光互补 (BiLC/BiFC) 和 BiLC/BiFC BRET,我们提供了趋化因子 (CXC 基序) 受体 4、非典型趋化因子受体 3、α 1a的证据肾上腺素受体和精氨酸加压素受体 1A 形成由 2-4 个不同的原体组成的异源寡聚体。我们表明,异源寡聚本身和与单个原体结合的配体调节激动剂诱导的与相互作用受体伙伴的信号转导器的耦合。我们的研究结果支持受体异源寡聚体形成具有不同于单个原体的分子信号传导特性的超分子机器的概念。这些发现为上下文依赖性受体功能的现象提供了一种机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号