Protein Expression and Purification ( IF 1.6 ) Pub Date : 2021-05-24 , DOI: 10.1016/j.pep.2021.105918 Tobias Heinks 1 , Anette Hettwer 2 , Christian Hiepen 3 , Christoph Weise 3 , Marcel Gorka 1 , Petra Knaus 3 , Thomas D Mueller 4 , Angelika Loidl-Stahlhofen 1
|
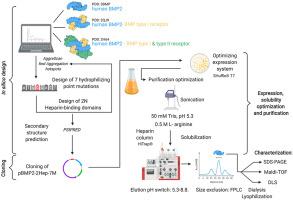
Bone morphogenetic protein 2 (BMP21) is a highly interesting therapeutic growth factor due to its strong osteogenic/osteoinductive potential. However, its pronounced aggregation tendency renders recombinant and soluble production troublesome and complex. While prokaryotic expression systems can provide BMP2 in large amounts, the typically insoluble protein requires complex denaturation-renaturation procedures with medically hazardous reagents to obtain natively folded homodimeric BMP2. Based on a detailed aggregation analysis of wildtype BMP2, we designed a hydrophilic variant of BMP2 additionally containing an improved heparin binding site (BMP2-2Hep-7M). Consecutive optimization of BMP2-2Hep-7M expression and purification enabled production of soluble dimeric BMP2-2Hep-7M in high yield in E. coli. This was achieved by a) increasing protein hydrophilicity via introducing seven point mutations within aggregation hot spots of wildtype BMP2 and a longer N-terminus resulting in higher affinity for heparin, b) by employing E. coli strain SHuffle® T7, which enables the structurally essential disulfide-bond formation in BMP2 in the cytoplasm, c) by using BMP2 variant characteristic soluble expression conditions and application of l-arginine as solubility enhancer. The BMP2 variant BMP2-2Hep-7M shows strongly attenuated although not completely eliminated aggregation tendency.
中文翻译:

基于计算机设计的可溶性 BMP2 变体的优化表达和纯化
骨形态发生蛋白 2 (BMP2 1 ) 是一种非常有趣的治疗性生长因子,因为它具有强大的成骨/骨诱导潜力。然而,其显着的聚集趋势使重组和可溶性生产变得麻烦和复杂。虽然原核表达系统可以提供大量的 BMP2,但通常不溶的蛋白质需要复杂的变性-复性程序和医学上危险的试剂才能获得天然折叠的同源二聚体 BMP2。基于对野生型 BMP2 的详细聚集分析,我们设计了一种 BMP2 的亲水变体,另外还含有改进的肝素结合位点 (BMP2-2Hep-7M)。BMP2-2Hep-7M 表达和纯化的连续优化使可溶性二聚体 BMP2-2Hep-7M 在大肠杆菌。这是通过以下方式实现的:a) 通过在野生型 BMP2 的聚集热点内引入 7 个点突变和更长的 N 端增加蛋白质亲水性,从而提高对肝素的亲和力,b) 通过使用大肠杆菌菌株 SHuffle® T7,这使得结构上能够在细胞质中 BMP2 中必需的二硫键形成,c) 通过使用 BMP2 变体特征可溶性表达条件和应用l-精氨酸作为溶解度增强剂。BMP2 变体 BMP2-2Hep-7M 显示出强烈减毒,但并未完全消除聚集趋势。


























 京公网安备 11010802027423号
京公网安备 11010802027423号