当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crystal structures of two camelid nanobodies raised against GldL, a component of the type IX secretion system from Flavobacterium johnsoniae
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2021-05-24 , DOI: 10.1107/s2053230x21005185 Thi Trang Nhung Trinh 1 , Anaïs Gaubert 2 , Pauline Melani 2 , Christian Cambillau 2 , Alain Roussel 2 , Philippe Leone 2
Acta Crystallographica Section F ( IF 1.072 ) Pub Date : 2021-05-24 , DOI: 10.1107/s2053230x21005185 Thi Trang Nhung Trinh 1 , Anaïs Gaubert 2 , Pauline Melani 2 , Christian Cambillau 2 , Alain Roussel 2 , Philippe Leone 2
Affiliation
|
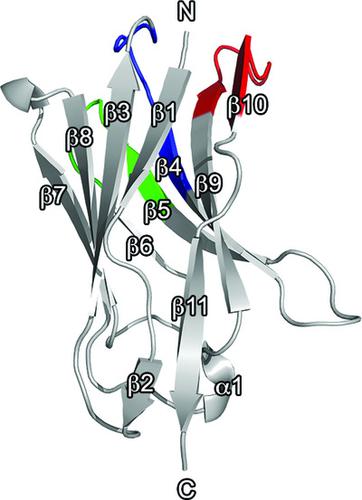
GldL is an inner-membrane protein that is essential for the function of the type IX secretion system (T9SS) in Flavobacterium johnsoniae. The complex that it forms with GldM is supposed to act as a new rotary motor involved in the gliding motility of the bacterium. In the context of structural studies of GldL to gain information on the assembly and function of the T9SS, two camelid nanobodies were selected, produced and purified. Their interaction with the cytoplasmic domain of GldL was characterized and their crystal structures were solved. These nanobodies will be used as crystallization chaperones to help in the crystallization of the cytoplasmic domain of GldL and could also help to solve the structure of the complex using molecular replacement.
中文翻译:

两种针对 GldL 的骆驼纳米体的晶体结构,GldL 是约氏黄杆菌 IX 型分泌系统的一个组成部分
GldL 是一种内膜蛋白,对约氏黄杆菌中 IX 型分泌系统 (T9SS) 的功能至关重要。它与 GldM 形成的复合物被认为是一种新的旋转马达,参与细菌的滑动运动。在 GldL 结构研究以获得 T9SS 的组装和功能信息的背景下,选择、生产和纯化了两种骆驼纳米抗体。表征了它们与 GldL 的细胞质结构域的相互作用,并解决了它们的晶体结构。这些纳米体将用作结晶伴侣,帮助 GldL 的细胞质结构域结晶,也可以帮助使用分子置换解决复合物的结构。
更新日期:2021-06-08
中文翻译:

两种针对 GldL 的骆驼纳米体的晶体结构,GldL 是约氏黄杆菌 IX 型分泌系统的一个组成部分
GldL 是一种内膜蛋白,对约氏黄杆菌中 IX 型分泌系统 (T9SS) 的功能至关重要。它与 GldM 形成的复合物被认为是一种新的旋转马达,参与细菌的滑动运动。在 GldL 结构研究以获得 T9SS 的组装和功能信息的背景下,选择、生产和纯化了两种骆驼纳米抗体。表征了它们与 GldL 的细胞质结构域的相互作用,并解决了它们的晶体结构。这些纳米体将用作结晶伴侣,帮助 GldL 的细胞质结构域结晶,也可以帮助使用分子置换解决复合物的结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号