Immunology and Cell Biology ( IF 4 ) Pub Date : 2021-05-20 , DOI: 10.1111/imcb.12461 Patrick M Gubser 1 , Ajithkumar Vasanthakumar 1, 2
|
Regulatory T (Treg) cells play a critical role in suppressing self-reactive T cells to preserve immune homeostasis.1 In the context of cancers, however, Treg cells dampen the function of anti-tumor immune cells to promote cancer progression.1 Altered cellular metabolism is one of the hallmarks of cancer cells. The high glycolytic activity and amino acid metabolism of cancer cells create a hostile tumor microenvironment (TME) that is depleted of glucose and amino acids. In addition, the high glucose utilization of tumors also makes the TME acidic due to the accumulation of lactic acid, a glycolytic by-product.2 Cellular metabolism and T cell development, maintenance and function are closely linked. CD8+ T cells are potent in killing tumor cells and share many metabolic similarities with cancer cells. The high glycolytic demand of CD8+ T cells for their effector function is detrimental to them in the TME where they are outcompeted by tumor cells for the available glucose.3 Besides limited glucose availability, lactate build-up in the TME also impedes the function of tumor infiltrating CD8+ T cells.4 Treg cells in contrast are traditionally known to rely on oxidative phosphorylation (OXPHOS) to generate energy.5 However, the link between altered TME and the increased suppressive activity of tumor infiltrating Treg cells has remained unknown until now.
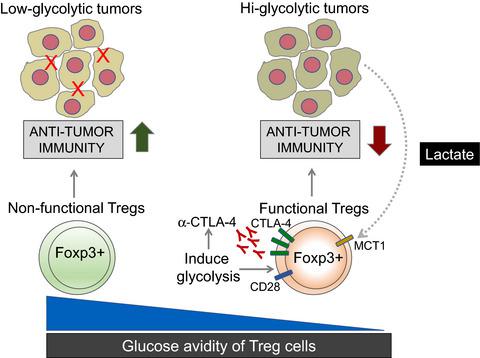
A string of papers published recently in the journal Nature revealed the unique metabolic features of tumor infiltrating Treg cells. In particular, two papers from Watson et al.6 and Zappazodi et al.7 investigated the impact of glucose availability and lactate build up on the suppressive function of Treg cells in the TME. Both these papers used distinct approaches to highlight the fact that Treg cells are functionally impaired in high concentrations of glucose. Watson et al. assessed the suppressive function of infiltrating Treg cells from tumors with different glycolytic potential. They discovered that the suppressive function of Treg cells was inversely proportional to the glycolytic ability of tumors (Figure 1). Interestingly, even in the same TME, using a fluorescent glucose tracer, Watson et al. were able to predict Treg suppressive function depending on their glucose avidity.6 Treg cells with high tracer uptake showed impaired suppressive function. In keeping with the functional defect, transcriptional profiling of tumor infiltrating Treg cells with high and low glucose avidity revealed downregulation of the genes responsible for immune suppression in Treg cells with high glucose avidity.6 Interestingly, low glucose avidity Treg cells showed upregulation of lactate dehydrogenase (ldha) and the monocarboxylate transporter MCT1 (Slc16a1).6 Lactate uptake is mediated through MCT1 and therefore possibly enables Treg cells to use it as an alternative energy source in highly glycolytic TME with high lactate concentrations. Tracing experiments with labeled lactate demonstrated that tumor Treg cells readily metabolized lactate into metabolites higher up in the glycolytic pathway via gluconeogenesis – which is the reversed form of glycolysis. Furthermore, Watson et al. ablated MCT1 in Treg cells using Foxp3Cre and implanted B16 melanoma tumors in these mice. Mice with Treg cells deficient for the lactate transporter MCT1, showed a lesser tumor burden supported by elevated activation of tumor infiltrating CD8+ T cells. Interestingly, intratumoral Treg cells from these mice became more glucose avid and developed an inflammatory phenotype by producing IFN-γ. Despite the functional defect in tumors, these mice did not develop autoimmunity suggesting context-specific usage of fuel source by Treg cells.6

Zappazodi et al. took a different approach to discover a destabilizing role of glycolysis in tumor infiltrating Treg cells. Using transcriptional profiling data from melanoma patients before and after ipilimumab (α-CTLA-4) treatment, these authors discovered that tumor glycolysis was negatively correlated with immune infiltration in tumors.7 Interestingly, this correlation diminished in ipilimumab treated patients but remained for LDHA and MCT1, suggesting that highly glycolytic tumors cannot be treated with ipilimumab single therapy. To fully understand the link between tumor glycolysis, CTLA-4 blockade and immune infiltration, Zappazodi et al. made LDHA deficient 4T1 mammary carcinoma cell lines. CTLA-4 blockade promoted the survival of mice carrying LDHA deficient tumors compared with controls with LDHA intact 4T1 tumors. In line with earlier studies, immune infiltrates in tumors of the same size revealed elevated numbers of CD8+ T cells and Treg cells in LDHA deficient tumors.7, 8 Interestingly, when LDHA deficient tumor bearing mice were treated with CTLA-4 blockade, the infiltrating Treg cells acquired an inflammatory phenotype akin to CD8+ T cells and produced IFN-γ. This inflammatory phenotype correlated with downregulation of CTLA-4 and CD25 in tumor infiltrating Treg cells.7 These authors discovered that CTLA-4 blockade promoted glycolysis in Treg cells leading to the acquisition of this inflammatory phenotype. In line with the findings from Watson et al., lactate reversed the inflammatory features. CD28 is known to induce glycolysis in T cells.9 By ablating CD28, these authors also showed that CTLA-4 mediated upregulation of glycolysis is dependent on CD28 signaling. To unequivocally demonstrate the inhibitory effect of glycolysis on Treg cells, Zappazodi et al. generated LDHA and Glut1 (glucose transporter) deficient Treg cells. When LDHA deficient tumors were implanted in these mice and treated with α-CTLA-4, Treg cells showed no more downregulation of CTLA-4 and CD25 as well as no upregulation of IFN-γ expression suggesting that truly glycolysis is responsible for the loss of Treg cell suppressive function. However, these Treg cells also lost functional fitness in the absence of LDHA or Glut1, suggesting a broader role for glycolysis in tumor infiltrating Treg cells.7
While these two papers highlight the negative role of glycolysis on the function of tumor infiltrating Treg cells, Watson et al. emphasize the usage of lactic acid as an alternate fuel source for tumor infiltrating Treg cells (Figure 1). Both studies show that forcing intratumoral Treg cells into glycolysis via either loss of lactate uptake (Watson et al.) or treatment with α-CTLA-4 antibodies (Zappazodi et al.) leads to a loss of their suppressive function (Figure 1). Despite the extraordinary attempt from these two groups to unravel the metabolic competition between tumors and Treg cells, there are several questions that remain unanswered. Whereas increased glycolysis is bad for Treg function, it remains unclear what impact dampened glycolysis has on intratumoral Treg cell function and maintenance. Zappazodi et al. used Glut1 and LDHA deficient Treg cells to demonstrate impaired glycolysis and short-term restoration of Treg cell function; however, the long-term maintenance was impaired. Given the importance of LDHA in the lactate metabolic pathway, it seems counterintuitive that deletion should promote Treg cell function. A possible explanation to this conundrum might come from the glucose–lactate axis proposed by Watson et al. Glycolysis is not a one-way street and gluconeogenesis, the reversed reaction of glycolysis, seems to be particularly important in intratumoral Treg cells. Depending on changes in glucose/lactate concentration, LDHA either converts pyruvate into lactate or the other way around.10 Effector T cells show high glucose uptake and lactate production,11 and activation of memory CD8+ T cells leads to rapid lactate production to support their effector function.12 These studies suggest a preference of glycolysis over gluconeogenesis in conventional CD4+ T cells and CD8+ T cells. Metabolic differences between T cell subsets therefore offer new opportunities to pharmacologically inhibit Treg cells specifically in the TME. While inhibiting lactate uptake or lactate conversion might look like an obvious target, it should be noted that the lactate transporter MCT1 also transports acetate, succinate, propionate and butyrate.13 It also remains to be determined how pharmacological inhibition/deletion of LDHA, an important part of the glycolytic pathway, is impacting the intratumoral CD4+ and CD8+ T cell responses. Dampening the effector response is likely to outweigh the beneficial effects of impaired Treg cell function in tumors. Pure gluconeogenesis enzymes seem to be more appropriate targets. However, Watson et al. could not show any beneficial effects on tumor growth by inhibiting phosphoenolpyruvate carboxylase (PEPCK), the rate limiting enzyme in gluconeogenesis.6 Further studies are required to define the specific metabolic requirements of tumor infiltrating T cells to therapeutically target them for the treatment of cancer.
中文翻译:

无糖:Treg 细胞避免葡萄糖以维持功能健康
调节性 T (Treg) 细胞在抑制自身反应性 T 细胞以保持免疫稳态方面发挥着关键作用。1然而,在癌症的背景下,Treg 细胞会抑制抗肿瘤免疫细胞促进癌症进展的功能。1细胞代谢改变是癌细胞的标志之一。癌细胞的高糖酵解活性和氨基酸代谢产生了一个缺乏葡萄糖和氨基酸的有害肿瘤微环境 (TME)。此外,由于糖酵解副产物乳酸的积累,肿瘤的高葡萄糖利用率也使 TME 呈酸性。2细胞代谢与T细胞的发育、维持和功能密切相关。CD8 +T 细胞能有效杀死肿瘤细胞,并且与癌细胞有许多代谢相似之处。CD8 + T 细胞对其效应子功能的高糖酵解需求对它们在 TME 中是有害的,在 TME 中,它们被肿瘤细胞竞争以获得可用的葡萄糖。3除了有限的葡萄糖可用性外,TME 中乳酸的堆积也会阻碍肿瘤浸润 CD8 + T 细胞的功能。4相比之下,传统上已知 Treg 细胞依赖氧化磷酸化 (OXPHOS) 来产生能量。5然而,直到现在,改变的 TME 与肿瘤浸润性 Treg 细胞抑制活性增加之间的联系仍然未知。
最近发表在《自然》杂志上的一系列论文揭示了肿瘤浸润性 Treg 细胞的独特代谢特征。特别是,沃森等人的两篇论文。6和 Zappazodi等。图 7研究了葡萄糖利用率和乳酸堆积对 TME 中 Treg 细胞抑制功能的影响。这两篇论文都使用了不同的方法来强调 Treg 细胞在高浓度葡萄糖中功能受损的事实。沃森等人. 评估了从具有不同糖酵解潜力的肿瘤中浸润 Treg 细胞的抑制功能。他们发现Treg细胞的抑制功能与肿瘤的糖酵解能力成反比(图1)。有趣的是,即使在同一个 TME 中,使用荧光葡萄糖示踪剂,Watson等。能够根据他们的葡萄糖亲和力预测 Treg 抑制功能。6具有高示踪剂摄取的 Treg 细胞显示出抑制功能受损。与功能缺陷保持一致,具有高和低葡萄糖亲和力的肿瘤浸润性 Treg 细胞的转录谱揭示了在具有高葡萄糖亲和力的 Treg 细胞中负责免疫抑制的基因的下调。6有趣的是,低葡萄糖亲和性 Treg 细胞显示出乳酸脱氢酶 ( ldha ) 和单羧酸转运蛋白 MCT1 ( Slc16a1 ) 的上调。6乳酸摄取是通过 MCT1 介导的,因此可能使 Treg 细胞能够将其用作具有高乳酸浓度的高糖酵解 TME 的替代能源。标记乳酸的追踪实验表明,肿瘤 Treg 细胞很容易通过糖异生(糖酵解的逆转形式)将乳酸代谢为糖酵解途径中更高的代谢物。此外,沃森等人。使用Foxp3 Cre消融 Treg 细胞中的 MCT1并在这些小鼠中植入 B16 黑色素瘤肿瘤。Treg 细胞缺乏乳酸转运蛋白 MCT1 的小鼠,肿瘤浸润 CD8 + T 细胞的活化升高,显示出较小的肿瘤负荷。有趣的是,来自这些小鼠的瘤内 Treg 细胞变得更热衷于葡萄糖,并通过产生 IFN-γ 产生炎症表型。尽管肿瘤存在功能缺陷,但这些小鼠并未产生自身免疫,这表明 Treg 细胞对燃料源的特定使用。6

扎帕佐迪等人。采取不同的方法来发现糖酵解在肿瘤浸润性 Treg 细胞中的不稳定作用。使用伊匹单抗(α-CTLA-4)治疗前后黑色素瘤患者的转录谱数据,这些作者发现肿瘤糖酵解与肿瘤中的免疫浸润呈负相关。7有趣的是,这种相关性在 ipilimumab 治疗的患者中减弱,但在 LDHA 和 MCT1 中仍然存在,这表明高糖酵解肿瘤不能用 ipilimumab 单一疗法治疗。为了充分了解肿瘤糖酵解、CTLA-4 阻断和免疫浸润之间的联系,Zappazodi等. 制造 LDHA 缺陷的 4T1 乳腺癌细胞系。与携带 LDHA 完整 4T1 肿瘤的对照相比,CTLA-4 阻断促进了携带 LDHA 缺陷肿瘤的小鼠的存活。与早期研究一致,相同大小肿瘤中的免疫浸润显示LDHA 缺陷肿瘤中 CD8 + T 细胞和 Treg 细胞数量增加。7, 8有趣的是,当用 CTLA-4 阻断剂治疗 LDHA 缺陷的荷瘤小鼠时,浸润的 Treg 细胞获得类似于 CD8 + T 细胞的炎症表型并产生 IFN-γ。这种炎症表型与肿瘤浸润性 Treg 细胞中 CTLA-4 和 CD25 的下调相关。7这些作者发现 CTLA-4 阻断促进了 Treg 细胞中的糖酵解,从而导致了这种炎症表型的获得。与 Watson等人的研究结果一致,乳酸逆转了炎症特征。已知 CD28 在 T 细胞中诱导糖酵解。9通过消融 CD28,这些作者还表明 CTLA-4 介导的糖酵解上调依赖于 CD28 信号传导。为了明确证明糖酵解对 Treg 细胞的抑制作用,Zappazodi等人. 产生 LDHA 和 Glut1(葡萄糖转运蛋白)缺陷的 Treg 细胞。当 LDHA 缺陷肿瘤被植入这些小鼠并用 α-CTLA-4 治疗时,Treg 细胞不再显示 CTLA-4 和 CD25 的下调以及 IFN-γ 表达的上调,这表明真正的糖酵解是造成Treg 细胞抑制功能。然而,在没有 LDHA 或 Glut1 的情况下,这些 Treg 细胞也失去了功能适应性,表明糖酵解在肿瘤浸润性 Treg 细胞中发挥着更广泛的作用。7
虽然这两篇论文强调了糖酵解对肿瘤浸润性 Treg 细胞功能的负面作用,但 Watson等人。强调使用乳酸作为肿瘤浸润性 Treg 细胞的替代燃料来源(图 1)。两项研究都表明,通过失去乳酸摄取(Watson等人)或用 α-CTLA-4 抗体处理(Zappazodi等人),迫使瘤内 Treg 细胞进入糖酵解状态.) 导致其抑制功能的丧失(图 1)。尽管这两组人为解开肿瘤和 Treg 细胞之间的代谢竞争做出了非凡的尝试,但仍有几个问题没有得到解答。尽管糖酵解增加对 Treg 功能不利,但仍不清楚抑制糖酵解对瘤内 Treg 细胞功能和维持有何影响。扎帕佐迪等. 使用 Glut1 和 LDHA 缺陷的 Treg 细胞证明糖酵解受损和 Treg 细胞功能的短期恢复;然而,长期维护受到损害。鉴于 LDHA 在乳酸代谢途径中的重要性,删除应该促进 Treg 细胞功能似乎违反直觉。这个难题的一个可能解释可能来自 Watson等人提出的葡萄糖-乳酸轴。糖酵解不是单向的,糖酵解的逆反应糖异生似乎在肿瘤内的 Treg 细胞中尤为重要。根据葡萄糖/乳酸浓度的变化,LDHA 要么将丙酮酸转化为乳酸,要么反过来。10效应 T 细胞显示出高葡萄糖摄取和乳酸产生,11和记忆 CD8 + T 细胞的激活导致乳酸的快速产生以支持其效应器功能。12这些研究表明,在常规 CD4 + T 细胞和 CD8 + T 细胞中,糖酵解优先于糖异生。因此,T 细胞亚群之间的代谢差异为特异性抑制 TME 中的 Treg 细胞提供了新的机会。虽然抑制乳酸摄取或乳酸转化似乎是一个明显的目标,但应注意乳酸转运蛋白 MCT1 还转运醋酸盐、琥珀酸盐、丙酸盐和丁酸盐。13LDHA(糖酵解途径的重要组成部分)的药理学抑制/缺失如何影响瘤内 CD4 +和 CD8 + T 细胞反应还有待确定。抑制效应反应可能会超过受损 Treg 细胞功能对肿瘤的有益影响。纯糖异生酶似乎是更合适的目标。然而,沃森等人。通过抑制磷酸烯醇式丙酮酸羧化酶 (PEPCK)(糖异生的限速酶),无法显示出对肿瘤生长的任何有益影响。6需要进一步的研究来确定肿瘤浸润性 T 细胞的特定代谢需求,以便在治疗上靶向它们以治疗癌症。


























 京公网安备 11010802027423号
京公网安备 11010802027423号