当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Atmospheric Oxidation of Propanesulfinic Acid Initiated by OH Radicals: Reaction Mechanism, Energetics, Rate Coefficients, and Atmospheric Implications
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-05-17 , DOI: 10.1021/acsearthspacechem.1c00062 Parandaman Arathala 1 , Rabi A. Musah 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2021-05-17 , DOI: 10.1021/acsearthspacechem.1c00062 Parandaman Arathala 1 , Rabi A. Musah 1
Affiliation
|
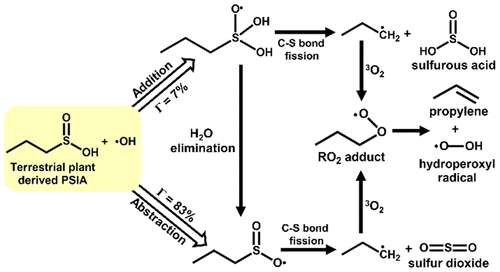
The atmospheric oxidation mechanism and energetics of propanesulfinic acid (CH3CH2CH2S(O)OH, PSIA) initiated by OH radicals have been investigated at the CCSD(T)/aug-cc-pVTZ//M06-2X/aug-cc-pVTZ level of theory. The PSIA + •OH reaction proceeds through (i) H-atom abstraction and (ii) •OH addition pathways. The calculated energies indicate that the barrier height for the abstraction of H-atom from the −OH moiety of PSIA leading to the formation of CH3CH2CH2S(O)2 + H2O is estimated to be −4.7 kcal mol–1 relative to that of the separated reactants. The rate coefficients were determined for all possible reaction paths by RRKM-ME calculations using Master equation solver for multienergy well reactions (Mesmer) code in the atmospherically relevant temperatures between 200 and 320 K and bath gas pressures between 0.1 and 10 atm. The calculated bimolecular rate coefficients suggest that the formation of CH3CH2CH2S(O)2 + H2O is predominant compared to the other possible reaction paths in the studied temperature range. The total rate coefficient for the PSIA + •OH reaction was found to be ∼8.40 × 10–11 cm3 molecule–1 s–1 at T = 298 K and P = 1 atm. In addition, branching ratios, thermochemical parameters, atmospheric lifetime, and global warming potentials were determined. Overall, the results indicate that the atmospheric removal of PSIA with •OH results in the formation of sulfur dioxide (SO2) from C–S single bond fission in the CH3–CH2–CH2–S(O)2 radical, which is formed by H-atom abstraction from the OH group of PSIA. Thus, the SO2 product does not originate from the direct elimination of SO2 from unimolecular dissociation of PSIA. The formed SO2, propylene (C3H6), sulfurous acid (H2SO3), and hydroperoxyl (HO2) radical are major products that may contribute to global warming and aerosol formation.
中文翻译:

由 OH 自由基引发的丙亚磺酸的大气氧化:反应机理、能量学、速率系数和大气影响
在CCSD(T)/aug-cc-pVTZ//M06-2X/aug上研究了OH自由基引发的丙亚磺酸(CH 3 CH 2 CH 2 S(O)OH, PSIA)的大气氧化机理和能量学-cc-pVTZ 理论水平。PSIA + • OH 反应通过 (i) H 原子提取和 (ii) • OH 添加途径进行。计算的能量表明,从 PSIA 的 -OH 部分提取 H 原子导致形成 CH 3 CH 2 CH 2 S(O) 2 + H 2 O的势垒高度估计为 -4.7 kcal mol –1相对于分离的反应物。在 200 至 320 K 之间的大气相关温度和 0.1 至 10 个大气压之间的浴气压力下,使用多能井反应的主方程求解器 (Mesmer) 代码,通过 RRKM-ME 计算确定所有可能反应路径的速率系数。计算出的双分子速率系数表明,在研究的温度范围内,与其他可能的反应路径相比,CH 3 CH 2 CH 2 S(O) 2 + H 2 O 的形成占主导地位。发现PSIA + • OH 反应的总速率系数为~8.40 × 10 –11 cm 3分子–1s –1在T = 298 K 和P = 1 atm。此外,还确定了支化率、热化学参数、大气寿命和全球变暖潜势。总体而言,结果表明,使用• OH大气去除 PSIA导致CH 3 -CH 2 -CH 2 -S(O) 2自由基中的C-S 单键裂变形成二氧化硫 (SO 2 ) ,它是通过从 PSIA 的 OH 基团中提取 H 原子形成的。因此,SO 2产物不是源自从PSIA的单分子解离直接消除SO 2。形成的 SO 2、丙烯 (C 3 H 6 )、亚硫酸 (H 2 SO 3 ) 和过氧化氢 (HO 2 ) 自由基是可能导致全球变暖和气溶胶形成的主要产物。
更新日期:2021-06-17
中文翻译:

由 OH 自由基引发的丙亚磺酸的大气氧化:反应机理、能量学、速率系数和大气影响
在CCSD(T)/aug-cc-pVTZ//M06-2X/aug上研究了OH自由基引发的丙亚磺酸(CH 3 CH 2 CH 2 S(O)OH, PSIA)的大气氧化机理和能量学-cc-pVTZ 理论水平。PSIA + • OH 反应通过 (i) H 原子提取和 (ii) • OH 添加途径进行。计算的能量表明,从 PSIA 的 -OH 部分提取 H 原子导致形成 CH 3 CH 2 CH 2 S(O) 2 + H 2 O的势垒高度估计为 -4.7 kcal mol –1相对于分离的反应物。在 200 至 320 K 之间的大气相关温度和 0.1 至 10 个大气压之间的浴气压力下,使用多能井反应的主方程求解器 (Mesmer) 代码,通过 RRKM-ME 计算确定所有可能反应路径的速率系数。计算出的双分子速率系数表明,在研究的温度范围内,与其他可能的反应路径相比,CH 3 CH 2 CH 2 S(O) 2 + H 2 O 的形成占主导地位。发现PSIA + • OH 反应的总速率系数为~8.40 × 10 –11 cm 3分子–1s –1在T = 298 K 和P = 1 atm。此外,还确定了支化率、热化学参数、大气寿命和全球变暖潜势。总体而言,结果表明,使用• OH大气去除 PSIA导致CH 3 -CH 2 -CH 2 -S(O) 2自由基中的C-S 单键裂变形成二氧化硫 (SO 2 ) ,它是通过从 PSIA 的 OH 基团中提取 H 原子形成的。因此,SO 2产物不是源自从PSIA的单分子解离直接消除SO 2。形成的 SO 2、丙烯 (C 3 H 6 )、亚硫酸 (H 2 SO 3 ) 和过氧化氢 (HO 2 ) 自由基是可能导致全球变暖和气溶胶形成的主要产物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号