当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nitrate reduction to ammonium: from CuO defect engineering to waste NOx-to-NH3 economic feasibility
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2021-5-11 , DOI: 10.1039/d1ee00594d Rahman Daiyan, Thanh Tran-Phu, Priyank Kumar, Kevin Iputera, Zizheng Tong, Joshua Leverett, Muhammad Haider Ali Khan, Ali Asghar Esmailpour, Ali Jalili, Maggie Lim, Antonio Tricoli, Ru-Shi Liu, Xunyu Lu, Emma Lovell, Rose Amal
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2021-5-11 , DOI: 10.1039/d1ee00594d Rahman Daiyan, Thanh Tran-Phu, Priyank Kumar, Kevin Iputera, Zizheng Tong, Joshua Leverett, Muhammad Haider Ali Khan, Ali Asghar Esmailpour, Ali Jalili, Maggie Lim, Antonio Tricoli, Ru-Shi Liu, Xunyu Lu, Emma Lovell, Rose Amal
|
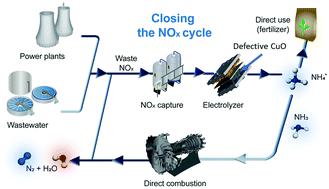
Critical to the feasibility of electrochemical reduction of waste NOx (NOxRR), as a sustainable pathway and to close the NOx cycle for the emerging NH3 economy, is the requirement of inexpensive, scalable and selective catalysts that can generate NH4+ with high yield, as indicated by our economic modelling. To this end, we carry out density functional theory (DFT) calculations to investigate the possible contribution of oxygen vacancy (OV) defects in NOxRR catalysis, discovering that an increase in defect density within CuO is leading to a decrease in adsorption energy for NO3− reactants. Using these findings as design guidelines, we develop defective CuO nanomaterials using flame spray pyrolysis (FSP) and mild plasma treatment, that can attain a NH4+ yield of 520 μmol cm−2 h−1 at a cell voltage of 2.2 V within a flow electrolyser with good stability over 10 h of operation. Through our mechanistic investigation, we establish the beneficial role of oxygen vacancy defects (with one free electron) in CuO for NOxRR and we reveal a direct correlation of oxygen vacancy density with the NH4+ yield, arising from improved NO3− adsorption, as evidenced from our theoretical calculations. Our findings on defect engineering to improve NH4+ yield and its economic feasibility display the potential of NOxRR as an alternative pathway to generate green NH3, which can also serve as an energy vector for the emerging hydrogen economy and close the NOx cycle.
中文翻译:

硝酸盐还原成铵:从CuO缺陷工程到将NOx转化为NH3的经济可行性
对于廉价,可扩展且选择性的能够生成NH 4的催化剂的要求,对于作为可持续途径并关闭NO x循环以实现新兴的NH 3经济,电化学还原废物NO x(NO x RR)的可行性至关重要。+具有产量高,为我们的经济模型来表示。为此,我们进行了密度泛函理论(DFT)计算,以研究NO x RR催化中氧空位(OV)缺陷的可能贡献,发现CuO中缺陷密度的增加导致吸附能量的降低。 NO 3 -反应物。使用这些发现作为设计指南,我们使用火焰喷雾热解(FSP)和温和的等离子体处理技术开发了有缺陷的CuO纳米材料,在2.2 V的电池电压下,NH 4 +的产率为520μmolcm -2 h -1电解槽在运行10小时后具有良好的稳定性。通过我们的调查机制,我们建立的CuO氧空位缺陷的有益作用(有一个自由电子)的NO X RR和我们揭示氧空位密度有直接的关系与NH 4 +的产量,产生从提高NO 3 -正如我们理论计算所证明的那样。我们在提高NH 4 +产量的缺陷工程方面的发现及其经济可行性表明,NO x RR作为产生绿色NH 3的替代途径的潜力,这也可以作为新兴氢经济和关闭NO x的能量载体循环。
更新日期:2021-05-17
中文翻译:

硝酸盐还原成铵:从CuO缺陷工程到将NOx转化为NH3的经济可行性
对于廉价,可扩展且选择性的能够生成NH 4的催化剂的要求,对于作为可持续途径并关闭NO x循环以实现新兴的NH 3经济,电化学还原废物NO x(NO x RR)的可行性至关重要。+具有产量高,为我们的经济模型来表示。为此,我们进行了密度泛函理论(DFT)计算,以研究NO x RR催化中氧空位(OV)缺陷的可能贡献,发现CuO中缺陷密度的增加导致吸附能量的降低。 NO 3 -反应物。使用这些发现作为设计指南,我们使用火焰喷雾热解(FSP)和温和的等离子体处理技术开发了有缺陷的CuO纳米材料,在2.2 V的电池电压下,NH 4 +的产率为520μmolcm -2 h -1电解槽在运行10小时后具有良好的稳定性。通过我们的调查机制,我们建立的CuO氧空位缺陷的有益作用(有一个自由电子)的NO X RR和我们揭示氧空位密度有直接的关系与NH 4 +的产量,产生从提高NO 3 -正如我们理论计算所证明的那样。我们在提高NH 4 +产量的缺陷工程方面的发现及其经济可行性表明,NO x RR作为产生绿色NH 3的替代途径的潜力,这也可以作为新兴氢经济和关闭NO x的能量载体循环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号