Journal of Environmental Chemical Engineering ( IF 7.7 ) Pub Date : 2021-05-15 , DOI: 10.1016/j.jece.2021.105670 Imran Majeed , Ayesha Arif , Muhammad Faizan , Mohd Adnan Khan , Muhammad Imran , Hassan Ali , Muhammad Amtiaz Nadeem , Muhammad Arif Nadeem
|
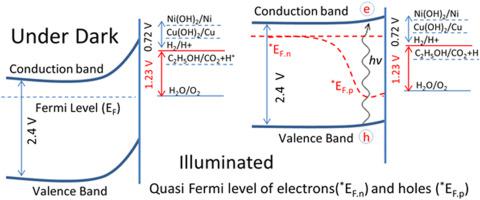
A series of composite photocatalysts based on CdS nanorods decorated with Cu/Ni hydroxides were prepared via deposition precipitation method at room temperature (total metal loading ≈ 3 wt.%). The performance of these photocatalysts was evaluated for H2 production through water dissociation reaction under direct sunlight irradiation. The 2.4Cu(OH)2 0.6Ni(OH)2/CdS photocatalyst with a total 3 wt.% nominal metal cocatalyst loading demonstrated an excellent hydrogen production activity of ~20 mmol h−1g−1, that is 2 and 20 times greater than the benchmark Au/CdS and pristine CdS photocatalysts, respectively. Detailed analyses based on UV-Visible diffuse reflectance spectroscopy (DRS), X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), steady state photoluminescence (PL) and energy dispersive spectroscopy (EDS) suggested cocatalysts Cu/Ni hydroxides were transformed into their metallic states during photoreaction. Cu° was produced by the transfer of electrons due to the as available position of CdS conduction band (E°=
0.6Ni(OH)2/CdS photocatalyst with a total 3 wt.% nominal metal cocatalyst loading demonstrated an excellent hydrogen production activity of ~20 mmol h−1g−1, that is 2 and 20 times greater than the benchmark Au/CdS and pristine CdS photocatalysts, respectively. Detailed analyses based on UV-Visible diffuse reflectance spectroscopy (DRS), X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), steady state photoluminescence (PL) and energy dispersive spectroscopy (EDS) suggested cocatalysts Cu/Ni hydroxides were transformed into their metallic states during photoreaction. Cu° was produced by the transfer of electrons due to the as available position of CdS conduction band (E°=  0.61 V) to surface Cu(OH)2 nanoclusters (Cu(OH)2/Cu°, E°=
0.61 V) to surface Cu(OH)2 nanoclusters (Cu(OH)2/Cu°, E°=  0.224 V) whereas Ni° was produced by the shift in conduction band level of CdS under continuous sunlight illumination (Ni(OH)2/Ni°, E°=
0.224 V) whereas Ni° was produced by the shift in conduction band level of CdS under continuous sunlight illumination (Ni(OH)2/Ni°, E°=  0.72 V). Based on these observations, the mechanism of hydrogen production from water splitting over Cu/Ni(hydroxides)-CdS was described.
0.72 V). Based on these observations, the mechanism of hydrogen production from water splitting over Cu/Ni(hydroxides)-CdS was described.
中文翻译:

CdS纳米棒负载的铜镍氢氧化物可在直接日光照射下制氢
在室温下(总金属负载量≈3 wt。%),通过沉积沉淀法制备了一系列以Cu / Ni氢氧化物修饰的CdS纳米棒为基础的复合光催化剂。通过在直接日光照射下通过水离解反应产生H 2来评估这些光催化剂的性能。具有总计3 wt。%的标称金属助催化剂负载量的2.4Cu(OH)2 0.6Ni(OH)2 / CdS光 催化剂显示出约20 mmol· h -1 g -1的优异制氢活性分别比基准Au / CdS和原始CdS光催化剂大2倍和20倍。基于紫外可见漫反射光谱(DRS),X射线衍射(XRD),透射电子显微镜(TEM),X射线光电子光谱(XPS),稳态光致发光(PL)和能量色散光谱(EDS)的详细分析)建议在光反应过程中助催化剂Cu / Ni氢氧化物转变成其金属态。由于CdS导带的有效位置(E°=
0.6Ni(OH)2 / CdS光 催化剂显示出约20 mmol· h -1 g -1的优异制氢活性分别比基准Au / CdS和原始CdS光催化剂大2倍和20倍。基于紫外可见漫反射光谱(DRS),X射线衍射(XRD),透射电子显微镜(TEM),X射线光电子光谱(XPS),稳态光致发光(PL)和能量色散光谱(EDS)的详细分析)建议在光反应过程中助催化剂Cu / Ni氢氧化物转变成其金属态。由于CdS导带的有效位置(E°=  0.61 V)到表面Cu(OH)2纳米团簇(Cu(OH)2 / Cu°,E°=
0.61 V)到表面Cu(OH)2纳米团簇(Cu(OH)2 / Cu°,E°=  0.224 )的电子转移产生了Cu° V),而Ni°是在连续的阳光照射下(Ni(OH)2 / Ni°,E°=
0.224 )的电子转移产生了Cu° V),而Ni°是在连续的阳光照射下(Ni(OH)2 / Ni°,E°=  0.72 V)通过CdS的导带能级变化产生的。基于这些观察,描述了水在Cu / Ni(氢氧化物)-CdS上分解产生氢气的机理。
0.72 V)通过CdS的导带能级变化产生的。基于这些观察,描述了水在Cu / Ni(氢氧化物)-CdS上分解产生氢气的机理。

























 京公网安备 11010802027423号
京公网安备 11010802027423号