Inorganica Chimica Acta Pub Date : 2021-05-14 , DOI: 10.1016/j.ica.2021.120451 Xiaoping Sun , Derrick R.J. Kolling , Amanda L. Smythers , Roger A. Deal
|
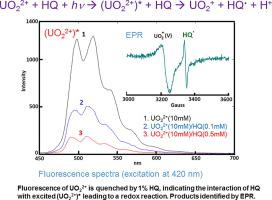
The aqueous mixtures of UO22+(VI) (as the nitrate salt) with benzene-1,4-diol (hydroquinone, HQ) 1,4-C6H4(OH)2 and those with oxalate C2O42− (as the sodium salt) exhibited broad absorptions at 350–400 nm in their UV–Vis spectra. The intensities of the absorptions for both the UO22+(VI)–HQ and UO22+(VI)–C2O42− mixtures, represented by the absorbance at 375 nm, were shown to be directly proportional to the molar concentrations of HQ and C2O42−, respectively. For each mixture, the absorbance was also found to be directly proportional to the molar concentration of UO22+(VI). The broad absorptions are characteristic of the charge-transfer (CT) bands from the electron-donor–acceptor (EDA) complexes formed between UO22+(VI) and HQ and between UO22+(VI) and C2O42−. Upon photolysis of the UO22+(VI)–HQ and UO22+(VI)–C2O42− mixtures, UO22+(VI) was found by electron paramagnetic resonance (EPR) to be reduced to UO2+(V) (g = 2.08) by HQ and C2O42−, respectively (CT reductions), and the hydroquinone radical 1,4-HOC6H4O. (HQ.) (g = 2.00) was identified simultaneously by EPR spectroscopy. Both HQ and C2O42− at the very low molar ratios of [HQ]/[UO22+] < 1/100 and [C2O42−]/[UO22+] < 1/100 were shown to quench the UO22+ luminescence (emission) for more than 60%. The quenching constants of HQ and C2O42− were determined to be 10,800 M−1 and 4,300 M−1, respectively, using the Stern-Volmer relationship. The results indicated a CT quenching mechanism, namely that the interactions of HQ and C2O42− with the excited *UO22+(VI) lead to transfer of a single electron from the quencher to *UO22+(VI) to reduce it to UO2+(V). This converts the light energy to chemical energy to quench the UO22+(VI) luminescence. Possible photochemical processes associated to the UO22+(VI)–HQ and UO22+(VI)–C2O42− redox reactions have been proposed on the basis of the UV–Vis, EPR, and luminescence spectroscopic studies.
中文翻译:

双氧铀的光化学电荷转移还原UO的调查2 2+(VI)到铀酰UO 2 +由苯-1,4-二醇(1,4-C(V)6 ħ 4(OH)2)和草酸( C 2 O 4 2−)的紫外可见,电子顺磁共振和发光光谱学
UO 2 2+(VI)(作为硝酸盐)与苯-1,4-二醇(对苯二酚,HQ)1,4-C 6 H 4(OH)2的水混合物和与草酸酯C 2 O 4的水混合物2-(作为钠盐)在UV-Vis光谱中在350-400 nm处显示出较宽的吸收率。UO 2 2+(VI)–HQ和UO 2 2+(VI)–C 2 O 4 2-混合物的吸收强度均以375 nm的吸光度表示,与吸光度成正比。 HQ和C 2 O 4 2-的摩尔浓度, 分别。对于每种混合物,还发现吸光度与UO 2 2+(VI)的摩尔浓度成正比。宽吸收是UO 2 2+(VI)和HQ之间以及UO 2 2+(VI)和C 2 O 4之间形成的电子给体-受体(EDA)配合物的电荷转移(CT)带的特征2−。在对UO 2 2+(VI)–HQ和UO 2 2+(VI)–C 2 O 4 2-的混合物进行光解后,通过电子顺磁共振(EPR)发现UO 2 2+(VI)被还原为O2 +(V)(G = 2.08)由HQ和C 2 Ò 4 2-,分别为(CT减少),和氢醌自由基1,4-HOC 6 H ^ 4 ö 。通过EPR光谱同时鉴定(HQ 。)(g = 2.00)。既HQ和C 2 ö 4 2-在[HQ]的非常低的摩尔比/ [UO 2 2+ ] <1/100和[C 2 Ò 4 2- ] / [UO 2 2+ ] <1/100结果显示,UU 2 2+的发光(发射)淬灭超过60%。HQ和C 2的猝灭常数使用斯特恩-沃尔默(Stern-Volmer)关系,将O 4 2-分别确定为10,800 M -1和4,300 M -1。结果表明CT猝灭机制,即HQ和C 2 O 4 2-与激发的* UO 2 2+(VI)的相互作用导致单个电子从猝灭剂转移到* UO 2 2+(VI ),将其降低到UO 2 +(V)。这将光能转换为化学能,以淬灭UO 2 2+(VI)发光。与UO 2 2+(VI)–HQ和UO 2相关的可能的光化学过程在UV-Vis,EPR和发光光谱研究的基础上,提出了2+(VI)–C 2 O 4 2-氧化还原反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号