Structure ( IF 5.7 ) Pub Date : 2021-05-13 , DOI: 10.1016/j.str.2021.04.011 Susan Kelso 1 , Stephen Orlicky 2 , Jonah Beenstock 2 , Derek F Ceccarelli 2 , Igor Kurinov 3 , Gerald Gish 2 , Frank Sicheri 4
|
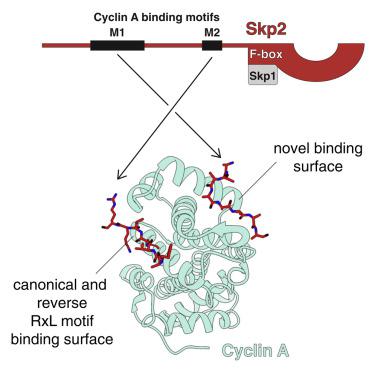
Skp2 and cyclin A are cell-cycle regulators that control the activity of CDK2. Cyclin A acts as an activator and substrate recruitment factor of CDK2, while Skp2 mediates the ubiquitination and subsequent destruction of the CDK inhibitor protein p27. The N terminus of Skp2 can interact directly with cyclin A but is not required for p27 ubiquitination. To gain insight into this poorly understood interaction, we have solved the 3.2 Å X-ray crystal structure of the N terminus of Skp2 bound to cyclin A. The structure reveals a bipartite mode of interaction with two motifs in Skp2 recognizing two discrete surfaces on cyclin A. The uncovered binding mechanism allows for a rationalization of the inhibitory effect of Skp2 on CDK2-cyclin A kinase activity toward the RxL motif containing substrates and raises the possibility that other intermolecular regulators and substrates may use similar non-canonical modes of interaction for cyclin targeting.
中文翻译:

Skp2的N末端与细胞周期蛋白A的二分结合
Skp2 和 cyclin A 是控制 CDK2 活性的细胞周期调节剂。Cyclin A 作为 CDK2 的激活剂和底物募集因子,而 Skp2 介导 CDK 抑制蛋白 p27 的泛素化和随后的破坏。Skp2 的 N 末端可以直接与细胞周期蛋白 A 相互作用,但不是 p27 泛素化所必需的。为了深入了解这种知之甚少的相互作用,我们已经解决了与细胞周期蛋白 A 结合的 Skp2 的 N 末端的 3.2 Å X 射线晶体结构。该结构揭示了与 Skp2 中的两个基序相互作用的二分模式,识别细胞周期蛋白上的两个离散表面一个。



























 京公网安备 11010802027423号
京公网安备 11010802027423号