当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Potential enantioselectivity of the hydrolysation and photolysation of the chiral agrochemical penthiopyrad in aquatic environments
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2021-5-5 , DOI: 10.1039/d1ew00098e Guangqian Yang 1 , Zhengyi Liu 1 , Tingting Lan 1 , Li Dou 1 , Kankan Zhang 1
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2021-5-5 , DOI: 10.1039/d1ew00098e Guangqian Yang 1 , Zhengyi Liu 1 , Tingting Lan 1 , Li Dou 1 , Kankan Zhang 1
Affiliation
|
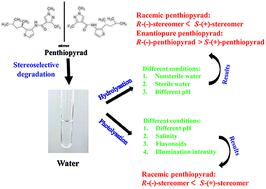
A liquid chromatography with tandem mass spectrometry method was developed and applied for the investigation of penthiopyrad photolysation and hydrolysation in different aqueous solutions, and the potential enantioselectivity of this chiral fungicide was further assessed. Good linearity was obtained (R2 > 0.99) for R-(−)- and S-(+)-penthiopyrad in different matrices and the respective limits of detection and quantitation of both enantiomers were 0.003 μg mL−1 and 0.01 μg mL−1. The most suitable illumination intensity for photolysing penthiopyrad was 400 W. Other factors such as pH, salinity and flavonoid also influenced penthiopyrad photolysation. In an acidic solution, the photolytic degradation rate of penthiopyrad enantiomers decreased due to their chemical structures and physicochemical characteristics. Different salinities affected penthiopyrad photolysation differently; acceleration occurred in solutions with salinities of 0.5% or 3.0% due to effective catalysis by photosensitive chloride radical anions, and when the salinity was 1.0% or 2.0%, the inhibitory effect of inorganic salt decreased the photolysation rate. The addition of different flavonoids could significantly inhibit the photolysation of penthiopyrad by decreasing degradation 5–597 times through photo-quenching effects. Under some conditions, the S-(+)-enantiomer was photolysed faster than its antipode, indicating that the photolysation of penthiopyrad was enantioselective. During the hydrolysation process, penthiopyrad degraded faster in the acidic solution than in the other two aqueous solutions with different pH levels, which was attributed to the hydrogen ion-induced catalytic reaction. In sterile/nonsterile natural water matrices, the hydrolysation rate of penthiopyrad enantiomers was also influenced by pH, in the degradation order of tap water (pH 7.1) > rain water (pH 7.3) > lake water (pH 8.8). Enantiopure enantiomers were hydrolysed more slowly than racemic enantiomers due to their mutual promotion effects. The configuration stability of penthiopyrad enantiomers was preserved during the degradation process. Similar enantioselectivities of the hydrolysation of penthiopyrad and preferential degradation of the S-(+)-enantiomer were observed in some aqueous solutions. The results could provide some data to more accurately demonstrate the enantioselective environmental fate and risk of penthiopyrad in aquatic environments.
中文翻译:

手性农药在水生环境中的水解和光解的潜在对映选择性
开发了一种采用串联质谱法的液相色谱法,并将其用于研究不同水溶液中戊硫吡py的光解和水解作用,并进一步评估了该手性杀菌剂的潜在对映选择性。良好的线性度得到(- [R 2 > 0.99)为- [R - ( - ) -和小号- (+) -在不同的矩阵和两种对映体的检测和定量的相应的限制吡噻菌胺为0.003微克毫升-1和0.01微克毫升- 1个。光解戊硫吡喃最合适的照明强度为400W。其他因素(例如pH值,盐度和类黄酮)也影响了戊硫吡咯的光解作用。在酸性溶液中,由于其化学结构和理化特性,对五苯并吡喃型对映体的光解速率降低。不同的盐度对戊硫吡photo的光解有不同的影响。由于光敏氯自由基阴离子的有效催化,在盐度为0.5%或3.0%的溶液中会发生加速,而当盐度为1.0%或2.0%时,无机盐的抑制作用会降低光解速率。添加不同的类黄酮可通过光猝灭作用将降解降低5–597倍,从而显着抑制戊硫吡喃的光解作用。在某些情况下,小号-(+)-对映体的光解速度快于其对映体的速度,这表明戊硫吡rad的光解是对映选择性的。在水解过程中,戊硫吡pen在酸性溶液中的降解速度快于其他两种pH值不同的水溶液,这归因于氢离子诱导的催化反应。在无菌/非无菌天然水基质中,对苯并吡喃对映体的水解速率也受到pH的影响,其降解顺序为自来水(pH 7.1)>雨水(pH 7.3)>湖泊水(pH 8.8)。对映体纯对映体由于相互促进作用,其水解速度比外消旋对映体慢。在降解过程中,保留了对苯并吡喃型对映异构体的构型稳定性。在某些水溶液中观察到S -(+)-对映体。结果可提供一些数据,以更准确地证明对映选择性的环境命运和在水生环境中对苯并吡喃的风险。
更新日期:2021-05-12
中文翻译:

手性农药在水生环境中的水解和光解的潜在对映选择性
开发了一种采用串联质谱法的液相色谱法,并将其用于研究不同水溶液中戊硫吡py的光解和水解作用,并进一步评估了该手性杀菌剂的潜在对映选择性。良好的线性度得到(- [R 2 > 0.99)为- [R - ( - ) -和小号- (+) -在不同的矩阵和两种对映体的检测和定量的相应的限制吡噻菌胺为0.003微克毫升-1和0.01微克毫升- 1个。光解戊硫吡喃最合适的照明强度为400W。其他因素(例如pH值,盐度和类黄酮)也影响了戊硫吡咯的光解作用。在酸性溶液中,由于其化学结构和理化特性,对五苯并吡喃型对映体的光解速率降低。不同的盐度对戊硫吡photo的光解有不同的影响。由于光敏氯自由基阴离子的有效催化,在盐度为0.5%或3.0%的溶液中会发生加速,而当盐度为1.0%或2.0%时,无机盐的抑制作用会降低光解速率。添加不同的类黄酮可通过光猝灭作用将降解降低5–597倍,从而显着抑制戊硫吡喃的光解作用。在某些情况下,小号-(+)-对映体的光解速度快于其对映体的速度,这表明戊硫吡rad的光解是对映选择性的。在水解过程中,戊硫吡pen在酸性溶液中的降解速度快于其他两种pH值不同的水溶液,这归因于氢离子诱导的催化反应。在无菌/非无菌天然水基质中,对苯并吡喃对映体的水解速率也受到pH的影响,其降解顺序为自来水(pH 7.1)>雨水(pH 7.3)>湖泊水(pH 8.8)。对映体纯对映体由于相互促进作用,其水解速度比外消旋对映体慢。在降解过程中,保留了对苯并吡喃型对映异构体的构型稳定性。在某些水溶液中观察到S -(+)-对映体。结果可提供一些数据,以更准确地证明对映选择性的环境命运和在水生环境中对苯并吡喃的风险。



























 京公网安备 11010802027423号
京公网安备 11010802027423号