Cell Stem Cell ( IF 23.9 ) Pub Date : 2021-05-10 , DOI: 10.1016/j.stem.2021.04.026 Ian J Penkala 1 , Derek C Liberti 2 , Joshua Pankin 3 , Aravind Sivakumar 3 , Madison M Kremp 4 , Sowmya Jayachandran 3 , Jeremy Katzen 5 , John P Leach 4 , Rebecca Windmueller 2 , Katharine Stolz 3 , Michael P Morley 4 , Apoorva Babu 4 , Su Zhou 6 , David B Frank 7 , Edward E Morrisey 8
|
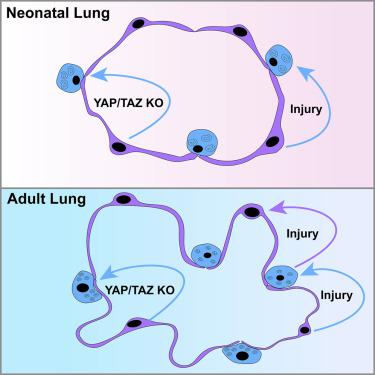
Regeneration of the architecturally complex alveolar niche of the lung requires precise temporal and spatial control of epithelial cell behavior. Injury can lead to a permanent reduction in gas exchange surface area and respiratory function. Using mouse models, we show that alveolar type 1 (AT1) cell plasticity is a major and unappreciated mechanism that drives regeneration, beginning in the early postnatal period during alveolar maturation. Upon acute neonatal lung injury, AT1 cells reprogram into alveolar type 2 (AT2) cells, promoting alveolar regeneration. In contrast, the ability of AT2 cells to regenerate AT1 cells is restricted to the mature lung. Unbiased genomic assessment reveals that this previously unappreciated level of plasticity is governed by the preferential activity of Hippo signaling in the AT1 cell lineage. Thus, cellular plasticity is a temporally acquired trait of the alveolar epithelium and presents an alternative mode of tissue regeneration in the postnatal lung.
中文翻译:

年龄依赖性肺泡上皮可塑性协调肺稳态和再生
肺结构复杂的肺泡生态位的再生需要对上皮细胞行为进行精确的时间和空间控制。受伤会导致气体交换表面积和呼吸功能永久性减少。使用小鼠模型,我们表明肺泡 1 型 (AT1) 细胞可塑性是驱动再生的主要且未被重视的机制,从肺泡成熟期间的产后早期开始。急性新生儿肺损伤后,AT1 细胞重新编程为肺泡 2 型 (AT2) 细胞,促进肺泡再生。相反,AT2 细胞再生 AT1 细胞的能力仅限于成熟肺。无偏见的基因组评估表明,这种以前未被重视的可塑性水平是由 AT1 细胞谱系中 Hippo 信号传导的优先活动决定的。因此,


























 京公网安备 11010802027423号
京公网安备 11010802027423号