当前位置:
X-MOL 学术
›
Acta Cryst. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Site-occupancy scheme in disordered Ca3RE2(BO3)4: a dependence on rare-earth (RE) ionic radius
Acta Crystallographica Section B ( IF 2.684 ) Pub Date : 2021-05-10 , DOI: 10.1107/s2052520621002328 Katarzyna M. Kosyl , Wojciech Paszkowicz , Roman Minikayev , Alexey N. Shekhovtsov , Miron B. Kosmyna , Maciej Chrunik , Andrew N. Fitch
Acta Crystallographica Section B ( IF 2.684 ) Pub Date : 2021-05-10 , DOI: 10.1107/s2052520621002328 Katarzyna M. Kosyl , Wojciech Paszkowicz , Roman Minikayev , Alexey N. Shekhovtsov , Miron B. Kosmyna , Maciej Chrunik , Andrew N. Fitch
|
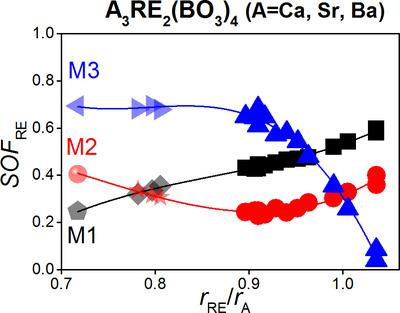
The structures of polycrystalline Ca3RE2(BO3)4 (RE = La, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Y; space group Pnma) orthoborates were determined using powder X-ray diffraction. Trends in the unit-cell dimensions and yet unreported trends in other structural properties (interatomic distances and the fractional occupation of three Ca/RE sites) for these compounds are demonstrated as a function of RE ionic radius. The unit-cell volume and a unit-cell parameter present a linear dependence, while the b and c unit-cell parameters change in a nonlinear manner. For the whole series, the RE atoms are present at all three cationic sites (labelled as M1, M2 and M3), but the fractional occupancies depend on the RE ionic radius. The small rare-earth atoms tend to enter mainly the M3 site; for the larger rare earths, the occupancy of this site decreases sharply. The occupancy of the M1 site by RE atoms is around 0.5 and tends to increase with increasing RE ionic radius. The M2 site is the least preferentially occupied by RE ions, but the occupancy discernibly increases with rising radius as well. These findings are assembled with properties of isostructural strontium and barium borates, allowing prediction of occupancy schemes for not yet investigated compounds from the A3RE2(BO3)4 (A = Ca, Ba, Sr).
中文翻译:

无序 Ca3RE2(BO3)4 中的占位方案:依赖于稀土 (RE) 离子半径
使用粉末 X 射线衍射确定多晶 Ca 3 RE 2 (BO 3 ) 4 (RE = La、Pr、Nd、Sm、Gd、Tb、Dy、Ho、Er、Y;空间群Pnma ) 原硼酸盐的结构。这些化合物的晶胞尺寸趋势和其他结构特性(原子间距离和三个 Ca/RE 位点的所占比例)的未报告趋势被证明是 RE 离子半径的函数。晶胞体积和一个晶胞参数呈现线性关系,而b和c ^晶胞参数以非线性方式变化。对于整个系列,RE 原子存在于所有三个阳离子位点(标记为M 1、M 2 和M 3),但分数占有率取决于 RE 离子半径。小的稀土原子倾向于主要进入M 3 位点;对于较大的稀土,该站点的占用率急剧下降。RE原子对M 1 位点的占有率约为0.5,并且随着RE离子半径的增加而增加。在中号2 位点最不优先被稀土离子占据,但占据率也随着半径的增加而明显增加。这些发现与同构的锶和硼酸钡的特性相结合,可以预测尚未研究的A 3 RE 2 (BO 3 ) 4 ( A = Ca、Ba、Sr)化合物的占有率方案。
更新日期:2021-06-07
中文翻译:

无序 Ca3RE2(BO3)4 中的占位方案:依赖于稀土 (RE) 离子半径
使用粉末 X 射线衍射确定多晶 Ca 3 RE 2 (BO 3 ) 4 (RE = La、Pr、Nd、Sm、Gd、Tb、Dy、Ho、Er、Y;空间群Pnma ) 原硼酸盐的结构。这些化合物的晶胞尺寸趋势和其他结构特性(原子间距离和三个 Ca/RE 位点的所占比例)的未报告趋势被证明是 RE 离子半径的函数。晶胞体积和一个晶胞参数呈现线性关系,而b和c ^晶胞参数以非线性方式变化。对于整个系列,RE 原子存在于所有三个阳离子位点(标记为M 1、M 2 和M 3),但分数占有率取决于 RE 离子半径。小的稀土原子倾向于主要进入M 3 位点;对于较大的稀土,该站点的占用率急剧下降。RE原子对M 1 位点的占有率约为0.5,并且随着RE离子半径的增加而增加。在中号2 位点最不优先被稀土离子占据,但占据率也随着半径的增加而明显增加。这些发现与同构的锶和硼酸钡的特性相结合,可以预测尚未研究的A 3 RE 2 (BO 3 ) 4 ( A = Ca、Ba、Sr)化合物的占有率方案。


























 京公网安备 11010802027423号
京公网安备 11010802027423号