Journal of Structural Biology ( IF 3 ) Pub Date : 2021-05-08 , DOI: 10.1016/j.jsb.2021.107742 Sophie L Winter 1 , Petr Chlanda 1
|
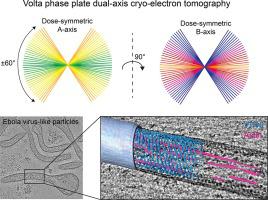
Cryo-electron tomography (cryo-ET) is a pivotal imaging technique for studying the structure of pleomorphic enveloped viruses and their interactions with the host at native conditions. Owing to the limited tilting range of samples with a slab geometry, electron tomograms suffer from so-called missing wedge information in Fourier space. In dual-axis cryo-ET, two tomograms reconstructed from orthogonally oriented tilt series are combined into a tomogram with improved resolution as the missing wedge information is reduced to a pyramid. Volta phase plate (VPP) allows to perform in-focus cryo-ET with high contrast transfer at low-resolution frequencies and thus its application may improve the quality of dual-axis tomograms. Here, we compare dual-axis cryo-ET with and without VPP on Ebola virus-like particles to visualize and segment viral and host cell proteins within the membrane-enveloped filamentous particles. Dual-axis VPP cryo-ET reduces the missing wedge information and ray artifacts arising from the weighted back-projection during tomogram reconstruction, thereby minimizing ambiguity in the analysis of crowded environments and facilitating 3D segmentation. We show that dual-axis VPP tomograms provide a comprehensive description of macromolecular organizations such as nucleocapsid assembly states, the distribution of glycoproteins on the viral envelope and asymmetric arrangements of the VP40 layer in non-filamentous regions of virus-like particles. Our data reveal actin filaments within virus-like particles in close proximity to the viral VP40 scaffold, suggesting a direct interaction between VP40 and actin filaments. Dual-axis VPP cryo-ET provides more complete 3D information at high contrast and allows for better interpretation of macromolecule interactions and pleomorphic organizations.
中文翻译:

埃博拉病毒样颗粒的双轴 Volta 相位板低温电子断层扫描揭示了肌动蛋白-VP40 的相互作用
冷冻电子断层扫描 (cryo-ET) 是一种关键的成像技术,用于研究多形性包膜病毒的结构及其在天然条件下与宿主的相互作用。由于具有平板几何形状的样品的倾斜范围有限,电子断层图在傅立叶空间中存在所谓的缺失楔形信息。在双轴低温 ET 中,从正交取向的倾斜序列重建的两个断层图像被组合成一个具有改进分辨率的断层图像,因为丢失的楔形信息被减少到一个金字塔。Volta 相位板 (VPP) 允许在低分辨率频率下执行具有高对比度传输的聚焦低温 ET,因此其应用可以提高双轴断层扫描的质量。这里,我们比较了在埃博拉病毒样颗粒上使用和不使用 VPP 的双轴低温 ET,以可视化和分割膜包裹的丝状颗粒内的病毒和宿主细胞蛋白。双轴 VPP cryo-ET 减少了断层图像重建过程中加权反投影产生的缺失楔形信息和射线伪影,从而最大限度地减少拥挤环境分析中的歧义并促进 3D 分割。我们表明,双轴 VPP 断层扫描提供了对大分子组织的全面描述,例如核衣壳组装状态、糖蛋白在病毒包膜上的分布以及 VP40 层在病毒样颗粒的非丝状区域中的不对称排列。我们的数据显示病毒样颗粒内的肌动蛋白丝靠近病毒 VP40 支架,表明VP40和肌动蛋白丝之间存在直接相互作用。双轴 VPP cryo-ET 以高对比度提供更完整的 3D 信息,并允许更好地解释大分子相互作用和多形性组织。


























 京公网安备 11010802027423号
京公网安备 11010802027423号