当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Spin-Regulated Inner-Sphere Electron Transfer Enables Efficient O—O Bond Activation in Nonheme Diiron Monooxygenase MIOX
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-05-06 , DOI: 10.1021/acscatal.1c00898 Jia Liu 1 , Peng Wu 1 , Shengheng Yan 1 , Yuanyuan Li 2 , Zexing Cao 1 , Binju Wang 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2021-05-06 , DOI: 10.1021/acscatal.1c00898 Jia Liu 1 , Peng Wu 1 , Shengheng Yan 1 , Yuanyuan Li 2 , Zexing Cao 1 , Binju Wang 1
Affiliation
|
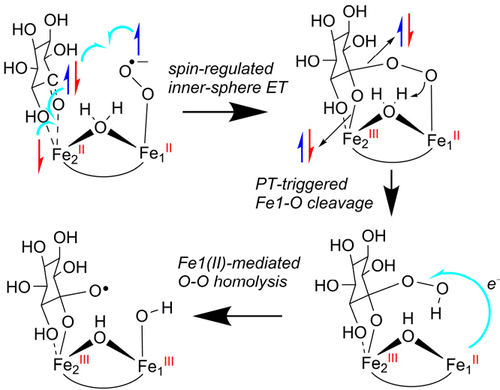
The combined molecular dynamics (MD) simulations and quantum mechanical/molecular mechanics (QM/MM) calculations have been performed to address the longstanding issue of the “dioxygen activation” by the nonheme diiron monooxygenase myo-inositol oxygenase (MIOX). MIOX utilizes a mixed-valence Fe2(III)Fe1(II) cluster for catalysis. It is well recognized that the Fe2(III) site is responsible for the substrate myo-inositol (MI) binding, while the Fe1(II) site is responsible for O2 binding and activation. However, it is enigmatic how the O–O bond of oxygen is reductively cleaved in the absence of additional reductants. In this study, we demonstrate a spin-regulated inner-sphere electron-transfer mechanism that is involved in the catalytic reactions of MIOX. Because of the Pauli principle and exchange-enhanced reactivity, the spin-regulated inner-sphere electron transfer enables the formation of an unprecedented Fe2(III)Fe1(II)-peroxyhemiketal intermediate that is responsible for the reductive O–O cleavage. In contrast to Fe1(III)-mediated O–O cleavage in the Fe2(II)Fe1(III)-peroxyhemiketal intermediate proposed previously, our calculations demonstrate that the proton transfer-triggered Fe1–O cleavage in Fe2(III)Fe1(II)-peroxyhemiketal intermediate is the most favorable pathway, leading to MI-OOH intermediate and the Fe1(II) species. The following Fe1(II)-mediated O–O homolysis in MI-OOH generates the substrate radical and Fe(III)–OH species, during which the Fe1(IV)═O intermediate would be bypassed. Thus, our calculations show that both Fe sites are cooperately involved in O2 activation in MIOX and such cooperation is well regulated by the spin-dependent inner-sphere electron transfer. These findings of O2 activation by MIOX may have far-reaching implications on other related nonheme diiron monooxygenases.
中文翻译:

自旋调节的内层电子转移可在非血红素二铁单加氧酶MIOX中实现有效的O-O键活化
已经进行了分子动力学(MD)模拟和量子力学/分子力学(QM / MM)的组合计算,以解决由非血红素二铁单加氧酶肌醇加氧酶(MIOX)引起的“双氧激活”的长期问题。MIOX利用混合价Fe2(III)Fe1(II)簇进行催化。众所周知,Fe2(III)位点负责底物肌醇(MI)的结合,而Fe1(II)位点负责O 2的作用。绑定和激活。然而,在没有其他还原剂的情况下,氧气的O-O键如何被还原裂解是个谜。在这项研究中,我们证明了自旋调节的内球电子转移机制参与MIOX的催化反应。由于泡利原理和交换增强的反应性,自旋调节的内球电子转移使得能够形成空前的Fe2(III)Fe1(II)-过氧半缩酮中间体,该中间体负责还原性O-O裂解。与先前提出的Fe2(II)Fe1(III)-过氧半水合物中间体中的Fe1(III)介导的O–O裂解相反,我们的计算表明,质子转移触发了Fe2(III)Fe1(II)的Fe1–O裂解。 )-过氧半水酮中间体是最有利的途径,导致MI-OOH中间体和Fe1(II)物种。在MI-OOH中,以下Fe1(II)介导的O-O均相分解产生了底物自由基和Fe(III)-OH,在此期间将绕过Fe1(IV)intermediateO中间体。因此,我们的计算表明两个铁位点共同参与了OMIOX中的2活化和这种合作受自旋依赖性内球电子转移的良好调控。MIOX激活O 2的这些发现可能对其他相关的非血红素二铁单加氧酶有深远的影响。
更新日期:2021-05-22
中文翻译:

自旋调节的内层电子转移可在非血红素二铁单加氧酶MIOX中实现有效的O-O键活化
已经进行了分子动力学(MD)模拟和量子力学/分子力学(QM / MM)的组合计算,以解决由非血红素二铁单加氧酶肌醇加氧酶(MIOX)引起的“双氧激活”的长期问题。MIOX利用混合价Fe2(III)Fe1(II)簇进行催化。众所周知,Fe2(III)位点负责底物肌醇(MI)的结合,而Fe1(II)位点负责O 2的作用。绑定和激活。然而,在没有其他还原剂的情况下,氧气的O-O键如何被还原裂解是个谜。在这项研究中,我们证明了自旋调节的内球电子转移机制参与MIOX的催化反应。由于泡利原理和交换增强的反应性,自旋调节的内球电子转移使得能够形成空前的Fe2(III)Fe1(II)-过氧半缩酮中间体,该中间体负责还原性O-O裂解。与先前提出的Fe2(II)Fe1(III)-过氧半水合物中间体中的Fe1(III)介导的O–O裂解相反,我们的计算表明,质子转移触发了Fe2(III)Fe1(II)的Fe1–O裂解。 )-过氧半水酮中间体是最有利的途径,导致MI-OOH中间体和Fe1(II)物种。在MI-OOH中,以下Fe1(II)介导的O-O均相分解产生了底物自由基和Fe(III)-OH,在此期间将绕过Fe1(IV)intermediateO中间体。因此,我们的计算表明两个铁位点共同参与了OMIOX中的2活化和这种合作受自旋依赖性内球电子转移的良好调控。MIOX激活O 2的这些发现可能对其他相关的非血红素二铁单加氧酶有深远的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号