Current Organic Synthesis ( IF 1.8 ) Pub Date : 2021-05-31 , DOI: 10.2174/1570179418666210113161550 Sarah Kappler 1 , Andreas Siebert 1 , Uli Kazmaier 1
|
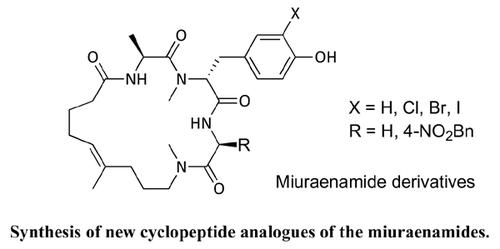
Introduction: Miuraenamides belong to natural marine compounds with interesting biological properties.
Materials and Methods: Miuraenamides initiate polymerization of monomeric actin and therefore show high cytotoxicity by influencing the cytoskeleton. New derivatives of the miuraenamides have been synthesized containing an N-methylated amide bond instead of the more easily hydrolysable ester in the natural products.
Results: Incorporation of an aromatic side chain onto the C-terminal amino acid of the tripeptide fragment also led to highly active new miuraenamides.
Conclusion: In this study, we showed that the ester bond of the natural product miuraenamide can be replaced by an N-methyl amide. The yields in the cyclization step were high and generally much better than with the corresponding esters. On the other hand, the biological activity of the new amide analogs was lower compared to the natural products, but the activity could significantly be increased by incorporation of a p-nitrophenyl group at the C-terminus of the peptide fragment.
中文翻译:

Miuraenamides 的新型环肽类似物的合成
简介:三脲烯酰胺属于天然海洋化合物,具有有趣的生物学特性。
材料和方法: Miuraenamides 引发单体肌动蛋白的聚合,因此通过影响细胞骨架显示出高细胞毒性。已经合成了含有 N-甲基化酰胺键的 miuraenamides 的新衍生物,而不是天然产物中更容易水解的酯。
结果:将芳族侧链并入三肽片段的 C 端氨基酸也导致了高活性的新 miuraenamides。
结论:在这项研究中,我们表明天然产物 miuraenamide 的酯键可以被 N-甲基酰胺取代。环化步骤的产率很高,并且通常比相应的酯好得多。另一方面,与天然产物相比,新酰胺类似物的生物活性较低,但通过在肽片段的 C 端掺入对硝基苯基,可以显着提高活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号