Current Organic Synthesis ( IF 1.8 ) Pub Date : 2021-07-31 , DOI: 10.2174/1570179417666201229163045 Reda Mohammed Keshk 1 , Batoul Mohamed Izzularab 1
|
Background: The continuous need for new anticancer drugs is never-ending task due to cancer resistance to the existing drugs.
Objective: This article aimed to design, synthesis, characterization, and anticancer evaluation of cyanopyridines, pyridopyrazolopyrimidines and pyridopyrazolotriazines.
Methods: Anticancer activity of the synthesized compounds was determined using MTT assay against three cancer cell lines, namely liver cancer cell line (HepG-2), pancreatic cancer cell line (PANC-1), non-small lung cancer cell line (A-549) and normal fibroblast.
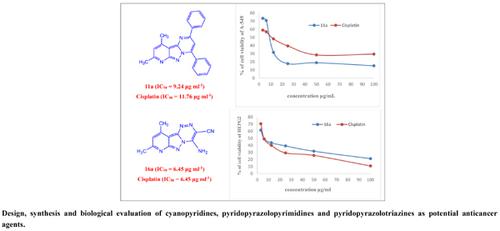
Results and Discussion: A series of 3-cyanopyridines (2a,b, 4, 5, 9), pyridopyrimidine (10), pyridopyrazolopyrimidines (11a-c, 12a,b, 18), pyrazolopyridine salt (13) and pyridopyrazolotriazines (16a,b) were synthesized from 3-cyano-4,6-dimethyl-2-pyridone. The synthesized compounds were evaluated in vitro for their anticancer activity and their chemical structures were determined by elemental analysis and spectroscopic data.
Conclusion: Some of the synthesized compounds showed remarkable anticancer activities, especially 11a exhibited superior potency to the reference drug cisplatin against A-549 (IC50 = 9.24 μg mL-1 compared to 11.76 μg mL-1 for reference drug) and was found to be safe (IC50 = 66 μg mL-1) for normal fibroblast. Furthermore, compound 16a displayed the highest activity among the tested compounds against HepG-2 (IC50 = 6.45 μg mL-1 equipotent to cisplatin) with the highest safety profile for normal fibroblast (IC50=113.97 μg mL-1).
中文翻译:

氰基吡啶、吡啶并吡唑并嘧啶和吡啶并吡唑并三嗪作为潜在抗癌剂的设计、合成和生物学评价
背景:由于癌症对现有药物的耐药性,对新抗癌药物的持续需求是永无止境的任务。
目的:本文旨在设计、合成、表征和抗癌评价氰基吡啶、吡啶并吡唑并嘧啶和吡啶并吡唑并三嗪。
方法:采用MTT法测定合成化合物对三种癌细胞系的抗癌活性,即肝癌细胞系(HepG-2)、胰腺癌细胞系(PANC-1)、非小肺癌细胞系(A- 549) 和正常的成纤维细胞。
结果与讨论:一系列 3-氰基吡啶(2a、b、4、5、9)、吡啶并嘧啶(10)、吡啶并吡唑并嘧啶(11a-c、12a、b、18)、吡唑并吡啶盐(13)和吡啶并吡唑并三嗪(16a, b) 由 3-氰基-4,6-二甲基-2-吡啶酮合成。合成的化合物在体外评估了它们的抗癌活性,并通过元素分析和光谱数据确定了它们的化学结构。
结论:一些合成的化合物显示出显着的抗癌活性,尤其是 11a 对 A-549 表现出优于参考药物顺铂的效力(IC50 = 9.24 μg mL-1,而参考药物为 11.76 μg mL-1),并且发现对正常成纤维细胞是安全的 (IC50 = 66 μg mL-1)。此外,化合物 16a 在测试化合物中对 HepG-2 的活性最高(IC50 = 6.45 μg mL-1 与顺铂等效),对正常成纤维细胞的安全性最高(IC50 = 113.97 μg mL-1)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号