Current Organic Synthesis ( IF 1.8 ) Pub Date : 2021-07-31 , DOI: 10.2174/1570179417666201218163435 Mzgin M Ayoob 1 , Awaz J Hussein 1 , Mohammed K Samad 1 , Necmi Dege 2 , Farouq E Hawaiz 1 , Shaaban K Mohamed 3 , Faiq H S Hussain 4
|
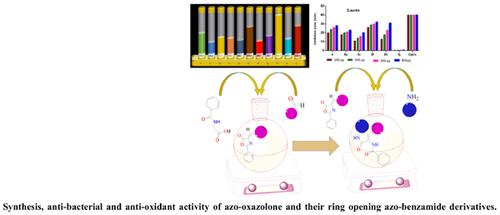
This article describes the controlled synthesis and characterization of azo oxazolone scaffold compounds containing multifunctional groups such as carbonyl group, imine and carbon-carbon double bond. The reaction of the azo-oxazolone with aromatic amines led to the ring-opening of the azo-oxazolone into the corresponding azo-benzamide derivatives in a short time (average 10 min), resulting in high yield (>90%). All newly synthesized compounds were characterized by the common spectral analysis such as UV, IR, 1H-NMR, 13CNMR, Elemental analysis and MS spectrometry.
Objective: The aim of the study was to synthesize new bioactive azo-benzamides by using azo-oxazolone as a synthon utilizing its ring-opening function.
Materials and Methods: Azo-benzamide derivatives were prepared in very good yield via ring-opening reaction of azo-oxazolone with aromatic amines in the presence of acetic acid under reflux for few minutes.
Results and Discussion: Chemical structures of the newly synthesized compounds were characterized by UV, IR, 1H-NMR, 13C-NMR, Elemental analysis and MS spectrometry.
Conclusion: The new azo-oxazolone 4 and azo-benzamide compounds 5a, 5c, 5f, 5h, 5j were screened against Escherichia coli as G(-ve) and Staphylococcus aureus as G(+ve) using ciprofloxacin as a standard. All compounds showed high inhibition potency against E-Coli but low inhibition for S-aureus. Compounds 4, 5c, and 5J showed more reactivity against E-coli.
Others: Also, the compounds were tested for their anti-oxidant activity by both DPPH and FRAP methods. The results showed that some compounds possessed moderate anti-oxidant activity in comparison to ascorbic acid as control, typically the compounds bearing OCH3 and OCH2CH3 groups.
中文翻译:

偶氮恶唑酮及其开环偶氮苯甲酰胺衍生物的合成、抗菌和抗氧化活性
本文介绍了含有羰基、亚胺和碳碳双键等多功能基团的偶氮恶唑酮支架化合物的可控合成和表征。偶氮恶唑酮与芳香胺的反应导致偶氮恶唑酮在短时间内(平均10分钟)开环成相应的偶氮苯甲酰胺衍生物,产率高(> 90%)。所有新合成的化合物均通过常见的光谱分析进行表征,如紫外、红外、1 H-NMR、13 CNMR、元素分析和 MS 光谱。
目的:本研究的目的是利用偶氮恶唑酮作为合成子,利用其开环功能合成新的具有生物活性的偶氮苯甲酰胺。
材料和方法:通过偶氮-恶唑酮与芳香胺在乙酸存在下回流几分钟的开环反应,以非常好的收率制备偶氮-苯甲酰胺衍生物。
结果与讨论:新合成化合物的化学结构通过UV、IR、1 H-NMR、13 C-NMR、元素分析和MS光谱表征。
结论: 以环丙沙星为标准,新的偶氮恶唑酮 4 和偶氮苯甲酰胺化合物 5a、5c、5f、5h、5j 被筛选为 G(-ve) 的大肠杆菌和 G(+ve) 的金黄色葡萄球菌。所有化合物均显示出对大肠杆菌的高抑制效力,但对金黄色葡萄球菌的抑制作用低。化合物 4、5c 和 5J 对大肠杆菌表现出更高的反应性。
其他:此外,还通过 DPPH 和 FRAP 方法测试了这些化合物的抗氧化活性。结果表明,与作为对照的抗坏血酸相比,一些化合物具有中等的抗氧化活性,通常带有OCH 3和OCH 2 CH 3基团的化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号