当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intermediates in allosteric equilibria of DnaK–ATP interactions with substrate peptides
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-05-05 , DOI: 10.1107/s2059798321002436 Wei Wang 1 , Wayne A Hendrickson 1
Acta Crystallographica Section D ( IF 2.2 ) Pub Date : 2021-05-05 , DOI: 10.1107/s2059798321002436 Wei Wang 1 , Wayne A Hendrickson 1
Affiliation
|
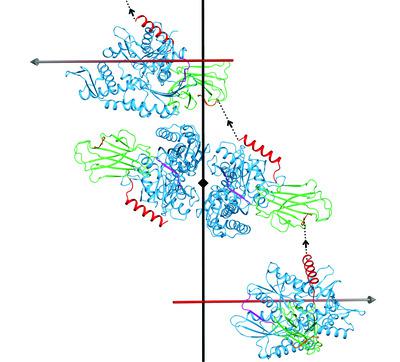
Hsp70 molecular chaperones facilitate protein disaggregation and proper folding through iterative cycles of polypeptide binding and release that are allosterically coupled to ATP binding and hydrolysis. Hsp70s are ubiquitous and highly conserved across all of life; they bind ATP at an N‐terminal nucleotide‐binding domain (NBD) and client peptides in the substrate‐binding domain (SBD). The NBD and SBD are connected by a highly conserved linker segment that is integrated into the NBD when ATP is bound but is flexible when the NBD is nucleotide‐free or bound with ADP. Allosteric coupling is lost when the linker is flexible, and the freed SBD binds peptide clients with high affinity. It was recently discovered that Hsp70–ATP is in an equilibrium between a restraining state (R) with little affinity for peptides and a low ATPase activity, and a stimulating state (S) that binds peptides efficiently, but with rapid kinetics, and has a relatively high ATPase activity. While attempting to characterize the S state, crystal structures of DnaK–ATP were obtained that demonstrate intrinsic Hsp70 plasticity that affects binding interactions with substrate peptides. These structures provide insights into intermediate states along transition pathways in the Hsp70 chaperone cycle.
中文翻译:

DnaK-ATP 与底物肽相互作用的变构平衡中的中间体
Hsp70 分子伴侣通过与 ATP 结合和水解变构耦合的多肽结合和释放的迭代循环促进蛋白质解聚和正确折叠。Hsp70 在整个生命体中普遍存在且高度保守;它们在 N 端核苷酸结合域 (NBD) 结合 ATP,并在底物结合域 (SBD) 结合客户肽。NBD 和 SBD 通过高度保守的接头片段连接,当 ATP 结合时,该接头片段整合到 NBD 中,但当 NBD 不含核苷酸或与 ADP 结合时,该接头片段是灵活的。当接头具有柔性时,变构偶联就会消失,并且释放的 SBD 以高亲和力结合肽客户。最近发现,Hsp70-ATP 处于对肽亲和力低且 ATP 酶活性较低的抑制状态 (R) 和有效结合肽但动力学快速且具有较低活性的刺激状态 (S) 之间的平衡。 ATP酶活性相对较高。在尝试表征 S 态时,获得了 DnaK-ATP 的晶体结构,证明了 Hsp70 的内在可塑性会影响与底物肽的结合相互作用。这些结构提供了对 Hsp70 伴侣循环中沿过渡途径的中间状态的深入了解。
更新日期:2021-05-06
中文翻译:

DnaK-ATP 与底物肽相互作用的变构平衡中的中间体
Hsp70 分子伴侣通过与 ATP 结合和水解变构耦合的多肽结合和释放的迭代循环促进蛋白质解聚和正确折叠。Hsp70 在整个生命体中普遍存在且高度保守;它们在 N 端核苷酸结合域 (NBD) 结合 ATP,并在底物结合域 (SBD) 结合客户肽。NBD 和 SBD 通过高度保守的接头片段连接,当 ATP 结合时,该接头片段整合到 NBD 中,但当 NBD 不含核苷酸或与 ADP 结合时,该接头片段是灵活的。当接头具有柔性时,变构偶联就会消失,并且释放的 SBD 以高亲和力结合肽客户。最近发现,Hsp70-ATP 处于对肽亲和力低且 ATP 酶活性较低的抑制状态 (R) 和有效结合肽但动力学快速且具有较低活性的刺激状态 (S) 之间的平衡。 ATP酶活性相对较高。在尝试表征 S 态时,获得了 DnaK-ATP 的晶体结构,证明了 Hsp70 的内在可塑性会影响与底物肽的结合相互作用。这些结构提供了对 Hsp70 伴侣循环中沿过渡途径的中间状态的深入了解。

























 京公网安备 11010802027423号
京公网安备 11010802027423号