当前位置:
X-MOL 学术
›
Adv. Theory Simul.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Energetic Arguments on the Microstructural Analysis in Ionic Liquids
Advanced Theory and Simulations ( IF 3.3 ) Pub Date : 2021-05-05 , DOI: 10.1002/adts.202100114 Takeshi Kobayashi 1 , Jens Smiatek 1 , Maria Fyta 1
Advanced Theory and Simulations ( IF 3.3 ) Pub Date : 2021-05-05 , DOI: 10.1002/adts.202100114 Takeshi Kobayashi 1 , Jens Smiatek 1 , Maria Fyta 1
Affiliation
|
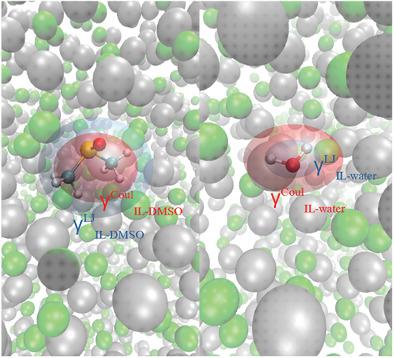
The interaction of ionic liquids (ILs) with additives or impurities is crucial for the performance of ILs in technological applications. In order to understand the interaction between these, an insight onto the microscopic arrangement of molecules is needed. As a representative case, 1-ethyl-3-methylimidazolium dicyanamide ([EMIM]+[DCA]−) mixtures are studied with dimethyl sulfoxide (DMSO) and water in bulk phase using molecular dynamics (MD) simulations. Different molar fractions of DMSO and water are studied to elucidate the influence of the molecular solute concentration on the structuring of the ILs. The results indicate that DMSO-[EMIM]+[DCA] and water-[EMIM]+[DCA]− mixtures are defined by quite distinct molecular interactions between the species. By increasing the DMSO or water concentration, in the former the solute molecules are repelled from the ions, while in the latter they accumulate closer to the ions. The differences arise from the weakening of the interactions between the ions imposed by the presence of the water molecules whereas DMSO promotes a strengthening of these interactions. Accordingly, this study provides a deep understanding of the IL behavior in combination with neutral solute molecules and allows for a proper selection of IL-solute combinations in view of specific technological applications.
中文翻译:

离子液体微观结构分析的能量论证
离子液体 (IL) 与添加剂或杂质的相互作用对于 IL 在技术应用中的性能至关重要。为了理解这些之间的相互作用,需要深入了解分子的微观排列。作为代表性案例,1-乙基-3-甲基咪唑鎓二氰胺([EMIM] + [DCA] -)混合物与二甲基亚砜(DMSO)和水在体相中使用分子动力学(MD)模拟进行研究。研究了不同摩尔分数的 DMSO 和水,以阐明分子溶质浓度对 IL 结构的影响。结果表明DMSO-[EMIM] + [DCA]和水-[EMIM] + [DCA] -混合物由物种之间完全不同的分子相互作用定义。通过增加 DMSO 或水的浓度,前者的溶质分子被离子排斥,而后者的溶质分子聚集在离离子更近的地方。差异源于水分子的存在强加的离子之间相互作用的减弱,而 DMSO 促进了这些相互作用的加强。Accordingly, this study provides a deep understanding of the IL behavior in combination with neutral solute molecules and allows for a proper selection of IL-solute combinations in view of specific technological applications.
更新日期:2021-07-14
中文翻译:

离子液体微观结构分析的能量论证
离子液体 (IL) 与添加剂或杂质的相互作用对于 IL 在技术应用中的性能至关重要。为了理解这些之间的相互作用,需要深入了解分子的微观排列。作为代表性案例,1-乙基-3-甲基咪唑鎓二氰胺([EMIM] + [DCA] -)混合物与二甲基亚砜(DMSO)和水在体相中使用分子动力学(MD)模拟进行研究。研究了不同摩尔分数的 DMSO 和水,以阐明分子溶质浓度对 IL 结构的影响。结果表明DMSO-[EMIM] + [DCA]和水-[EMIM] + [DCA] -混合物由物种之间完全不同的分子相互作用定义。通过增加 DMSO 或水的浓度,前者的溶质分子被离子排斥,而后者的溶质分子聚集在离离子更近的地方。差异源于水分子的存在强加的离子之间相互作用的减弱,而 DMSO 促进了这些相互作用的加强。Accordingly, this study provides a deep understanding of the IL behavior in combination with neutral solute molecules and allows for a proper selection of IL-solute combinations in view of specific technological applications.


























 京公网安备 11010802027423号
京公网安备 11010802027423号