当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Measurement and Data Correlation of 4-Chlorophenoxyacetic Acid in 13 Monosolvents at Temperatures from 283.15 to 328.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-05-05 , DOI: 10.1021/acs.jced.1c00153 Zhenjie Gao 1, 2 , Jiawei Lin 1, 2 , Peng Shi 1, 2 , Zhenkai Cen 1, 2 , Zhixu Li 1, 2 , Dandan Han 1, 2 , Junbo Gong 1, 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2021-05-05 , DOI: 10.1021/acs.jced.1c00153 Zhenjie Gao 1, 2 , Jiawei Lin 1, 2 , Peng Shi 1, 2 , Zhenkai Cen 1, 2 , Zhixu Li 1, 2 , Dandan Han 1, 2 , Junbo Gong 1, 2
Affiliation
|
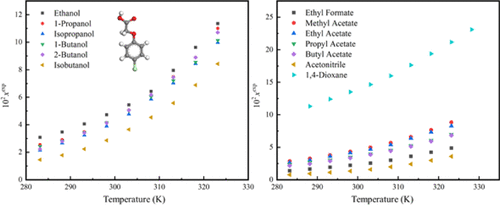
The solubility of 4-chlorophenoxyacetic acid (4-CPA) in 13 monosolvents including ethanol, 1-propanol, isopropanol, 1-butanol, 2-butanol, isobutanol, ethyl formate, methyl acetate, ethyl acetate, propyl acetate, butyl acetate, acetonitrile, and 1,4-dioxane was measured by a gravimetric method from 283.15 to 328.15 K at an atmospheric pressure of 0.1013 MPa. The solubility of 4-CPA increased with temperature and followed the order 1,4-dioxane > alcohols > esters > acetonitrile at all experimental temperatures. The solvent effects, especially solvent polarity, hydrogen bond donor propensity, and hydrogen bond acceptor propensity, were used to analyze the solubility of 4-CPA in different solvents. The solubility basically follows the rule “like dissolves like” and has a higher value in solvents with strong hydrogen bond donor propensity and hydrogen bond acceptor propensity. Besides, the modified Apelblat equation, λh equation, and van’t Hoff equation were applied to correlate the measured solubility data. The average relative deviation (ARD) values showed that the correlation results of the three models agree well with the experimental data.
中文翻译:

4-氯苯氧乙酸在 283.15 至 328.15 K 温度下在 13 种单溶剂中的溶解度测量和数据相关性
4-氯苯氧乙酸(4-CPA)在乙醇、1-丙醇、异丙醇、1-丁醇、2-丁醇、异丁醇、甲酸乙酯、乙酸甲酯、乙酸乙酯、乙酸丙酯、乙酸丁酯、乙腈等13种单溶剂中的溶解度和 1,4-二恶烷通过重量法在 0.1013 MPa 的大气压下从 283.15 到 328.15 K 测量。4-CPA 的溶解度随着温度的升高而增加,并且在所有实验温度下都遵循 1,4-二恶烷 > 醇 > 酯 > 乙腈的顺序。溶剂效应,特别是溶剂极性、氢键供体倾向和氢键受体倾向,用于分析 4-CPA 在不同溶剂中的溶解度。溶解度基本遵循“相似相溶”的规律,在具有强氢键供体倾向和氢键受体倾向的溶剂中具有较高的值。此外,修正的 Apelblat 方程,λh方程和 van't Hoff 方程用于关联测得的溶解度数据。平均相对偏差(ARD)值表明三种模型的相关结果与实验数据吻合较好。
更新日期:2021-06-10
中文翻译:

4-氯苯氧乙酸在 283.15 至 328.15 K 温度下在 13 种单溶剂中的溶解度测量和数据相关性
4-氯苯氧乙酸(4-CPA)在乙醇、1-丙醇、异丙醇、1-丁醇、2-丁醇、异丁醇、甲酸乙酯、乙酸甲酯、乙酸乙酯、乙酸丙酯、乙酸丁酯、乙腈等13种单溶剂中的溶解度和 1,4-二恶烷通过重量法在 0.1013 MPa 的大气压下从 283.15 到 328.15 K 测量。4-CPA 的溶解度随着温度的升高而增加,并且在所有实验温度下都遵循 1,4-二恶烷 > 醇 > 酯 > 乙腈的顺序。溶剂效应,特别是溶剂极性、氢键供体倾向和氢键受体倾向,用于分析 4-CPA 在不同溶剂中的溶解度。溶解度基本遵循“相似相溶”的规律,在具有强氢键供体倾向和氢键受体倾向的溶剂中具有较高的值。此外,修正的 Apelblat 方程,λh方程和 van't Hoff 方程用于关联测得的溶解度数据。平均相对偏差(ARD)值表明三种模型的相关结果与实验数据吻合较好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号