当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fe1N4–O1 site with axial Fe–O coordination for highly selective CO2 reduction over a wide potential range
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2021-4-27 , DOI: 10.1039/d1ee00569c Zhiqiang Chen 1, 2, 3, 4 , Aijian Huang 1, 2, 3, 4, 5 , Ke Yu 1, 2, 3, 4 , Tingting Cui 1, 2, 3, 4 , Zewen Zhuang 1, 2, 3, 4 , Shoujie Liu 4, 6, 7 , Jianzhan Li 1, 2, 3, 4 , Renyong Tu 1, 2, 3, 4 , Kaian Sun 1, 2, 3, 4 , Xin Tan 1, 2, 3, 4 , Jiaqi Zhang 1, 2, 3, 4 , Di Liu 1, 2, 3, 4 , Yu Zhang 1, 2, 3, 4 , Peng Jiang 1, 2, 3, 4 , Yuan Pan 1, 2, 3, 4 , Chen Chen 1, 2, 3, 4 , Qing Peng 1, 2, 3, 4 , Yadong Li 1, 2, 3, 4
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2021-4-27 , DOI: 10.1039/d1ee00569c Zhiqiang Chen 1, 2, 3, 4 , Aijian Huang 1, 2, 3, 4, 5 , Ke Yu 1, 2, 3, 4 , Tingting Cui 1, 2, 3, 4 , Zewen Zhuang 1, 2, 3, 4 , Shoujie Liu 4, 6, 7 , Jianzhan Li 1, 2, 3, 4 , Renyong Tu 1, 2, 3, 4 , Kaian Sun 1, 2, 3, 4 , Xin Tan 1, 2, 3, 4 , Jiaqi Zhang 1, 2, 3, 4 , Di Liu 1, 2, 3, 4 , Yu Zhang 1, 2, 3, 4 , Peng Jiang 1, 2, 3, 4 , Yuan Pan 1, 2, 3, 4 , Chen Chen 1, 2, 3, 4 , Qing Peng 1, 2, 3, 4 , Yadong Li 1, 2, 3, 4
Affiliation
|
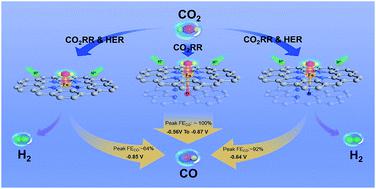
On the path to deploying the electrochemical CO2 reduction reaction (CO2RR) to CO, the narrow potential range under the high Faraday efficiency of CO (FECO) still blocks its ultimate practical viability. Engineering the electronic structure via heteroatom doping is supposed to be efficient. However, a feasible synthesis and precise control are still challenging. Here, we propose a fast-pyrolyzing and controllable-activation strategy to construct an O-rich carbonaceous support and atomically dispersed FeN4 site with axial O coordination (Fe1N4–O1), which was confirmed by aberration-corrected electron microscopy and X-ray absorption spectroscopy. A wide potential range of 310 mV (−0.56 V to −0.87 V vs. RHE) could be achieved when FECO was continuously maintained at nearly 100%, which exceeded the existing results to the best of our knowledge. DFT calculations revealed that the superior performance originated from the axial O-coordination induced electronic localization enhancement, which could facilitate the desorption of CO and increase the energy barrier for the competitive hydrogen evolution reaction.
中文翻译:

具有轴向Fe–O配位的Fe1N4–O1站点可在很宽的潜在范围内高度选择性地减少CO2
在将电化学CO 2还原反应(CO 2 RR)部署为CO的路径上,在高法拉第效率(FE CO)下,狭窄的电位范围仍然阻碍了其最终的实用性。通过杂原子掺杂工程电子结构被认为是有效的。然而,可行的合成和精确控制仍具有挑战性。在这里,我们提出了一种快速热解和可控制的活化策略,以构建具有轴向O配位的富O碳质载体和原子分散的FeN 4位点(Fe 1 N 4 –O 1),这是通过像差校正电子显微镜和X射线吸收光谱法确认的。当FE CO连续保持接近100%时,可以实现310 mV的宽电位范围(相对于RHE为-0.56 V至-0.87 V ),据我们所知,这超过了现有结果。DFT计算表明,优异的性能源自轴向O配位诱导的电子定位增强,这可以促进CO的解吸并增加竞争性放氢反应的能垒。
更新日期:2021-05-06
中文翻译:

具有轴向Fe–O配位的Fe1N4–O1站点可在很宽的潜在范围内高度选择性地减少CO2
在将电化学CO 2还原反应(CO 2 RR)部署为CO的路径上,在高法拉第效率(FE CO)下,狭窄的电位范围仍然阻碍了其最终的实用性。通过杂原子掺杂工程电子结构被认为是有效的。然而,可行的合成和精确控制仍具有挑战性。在这里,我们提出了一种快速热解和可控制的活化策略,以构建具有轴向O配位的富O碳质载体和原子分散的FeN 4位点(Fe 1 N 4 –O 1),这是通过像差校正电子显微镜和X射线吸收光谱法确认的。当FE CO连续保持接近100%时,可以实现310 mV的宽电位范围(相对于RHE为-0.56 V至-0.87 V ),据我们所知,这超过了现有结果。DFT计算表明,优异的性能源自轴向O配位诱导的电子定位增强,这可以促进CO的解吸并增加竞争性放氢反应的能垒。


























 京公网安备 11010802027423号
京公网安备 11010802027423号