Journal of the Taiwan Institute of Chemical Engineers ( IF 5.7 ) Pub Date : 2021-05-05 , DOI: 10.1016/j.jtice.2021.04.005 M. Clotilde Apua , Mapilane S. Madiba
|
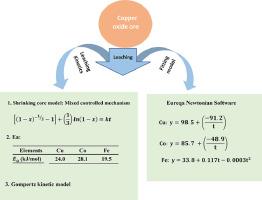
The results of experimental investigation on the study of leaching kinetics of copper oxide ore in sulphuric acid solution were discussed. The effect of time, pH, stirring speed and temperature on the extend of dissolution was investigated. The XRD analysis indicated that copper is mainly in the form of malachite mineral CuCO3(OH)2. The elemental analysis by XRF showed that the copper oxide ore is composed mainly of 10.07% Cu, 0.71% Co, 4.12% Fe, 24.29% Si, 5.20% Mg, 2.50% Al, 1.00% Ca and 0.48% Mn. Results of dissolution studies showed that the copper oxide ore leaching in H2SO4 solution increase with increasing time, pH, stirring speed and temperature at fixed H2SO4 concentration of 0.77 M. The study showed that 97.02%, 85.41% and 39.64% of copper, cobalt and iron were, respectively, leached by H2SO4 at 70 °C, pH value 1 within 60 min. The important part of this study was to analyse the mechanism and to develop fitting models for leaching process of copper oxide ore by sulphuric acid solution. In understanding the mechanism of dissolution, the reaction rate controlling factor and the order of reaction are of interest. The experimental data were best fitted by mixed controlled mechanism (diffusion and chemical reaction). The corresponding activation energies were calculated to be 24.0 kJ/mol for copper, 28.1 kJ/mol for cobalt and 19.5 kJ/mol for iron. The reaction order was determined to be Gompertz kinetic model. In developing fitting models for the prediction of leaching of copper, cobalt and iron, Eureqa Newtonian software was used. Results confirmed that the suggested models can be effective tools to predict the behaviour and outputs of the dissolution process.
中文翻译:

硫酸中氧化铜矿中元素萃取的浸出动力学和预测模型
讨论了研究氧化铜矿在硫酸溶液中浸出动力学的实验研究结果。研究了时间,pH,搅拌速度和温度对溶解时间的影响。XRD分析表明,铜主要以孔雀石矿物CuCO 3(OH)2的形式存在。XRF的元素分析表明,氧化铜矿石主要由10.07%的Cu,0.71%的Co,4.12%的Fe,24.29%的Si,5.20%的Mg,2.50%的Al,1.00%的Ca和0.48%的Mn组成。溶出度研究结果表明,在固定的H 2 SO 4下,H 2 SO 4溶液中氧化铜矿的浸出随着时间,pH,搅拌速度和温度的增加而增加。浓度为0.77M。研究表明,H 2 SO 4分别浸出了97.02%,85.41%和39.64%的铜,钴和铁。在70°C下,在60分钟内pH值为1。本研究的重要部分是分析硫酸溶液浸出氧化铜矿的机理和建立拟合模型。在理解溶解机理时,反应速率控制因素和反应顺序是令人关注的。通过混合控制机制(扩散和化学反应)可以最好地拟合实验数据。计算出相应的活化能:铜为24.0 kJ / mol,钴为28.1 kJ / mol,铁为19.5 kJ / mol。反应顺序确定为Gompertz动力学模型。在开发用于预测铜,钴和铁浸出量的拟合模型时,使用了Eureqa Newtonian软件。


























 京公网安备 11010802027423号
京公网安备 11010802027423号