Chemical Engineering Science ( IF 4.7 ) Pub Date : 2021-05-05 , DOI: 10.1016/j.ces.2021.116731 T.O. Bello , A.E. Bresciani , C.A.O. Nascimento , R.M.B. Alves
|
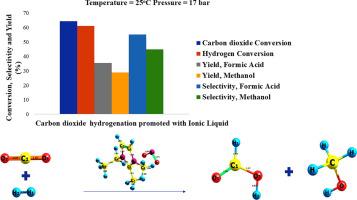
Direct hydrogenation of carbon dioxide (CO2) to formic acid is unfavorable thermodynamically, which makes its production limited. In this study, a thermodynamic analysis of CO2 hydrogenation to binary product systems of methanol and formic acid promoted by ionic liquid (IL) (1-ethyl-2,3-dimethylimidazolium nitrite, ([Edmim][NO2]) is presented. The analysis is conducted in Aspen Plus using the Gibbs energy minimization approach combined with a vapor–liquid equilibrium (VLE) for the solvation of CO2 in IL. It is demonstrated that solvating CO2 in ILs is an attractive alternative to overcome the thermodynamic difficulty associated with the product yield, especially formic acid. The [Edmim][NO2] promoted system is very effective for the simultaneous production of formic acid and methanol at 25 °C and 17 bar with a yield of 35% formic acid and 30% methanol at a CO2/H2/IL ratio of 1/2/2. The results show a marked improvement in the yield of formic acid to other previously conducted studies on formic acid production.
中文翻译:

二氧化碳加氢成甲酸和甲醇的热力学分析
将二氧化碳(CO 2)直接氢化成甲酸在热力学上是不利的,这使其生产受到限制。在这项研究中,提出了由离子液体(IL)(1-乙基-2,3-二甲基咪唑鎓亚硝酸盐([Edmim] [NO 2 ])促进的CO 2加氢成甲醇和甲酸的二元产物体系的热力学分析。。该分析是在Aspen Plus中使用吉布斯能量最小化方法结合气液平衡(VLE)进行IL中的CO 2溶剂化的结果,这表明在ILs中使CO 2溶剂化是克服热力学的一种有吸引力的替代方法[Edmim] [NO 2]促进体系对于在25°C和17 bar下同时生产甲酸和甲醇非常有效,CO 2 / H 2 / IL比为1/2/2时,甲酸和甲醇的收率分别为35%和30%。结果表明,相对于先前进行的其他有关甲酸生产的研究,甲酸的收率显着提高。



























 京公网安备 11010802027423号
京公网安备 11010802027423号