Biomaterials Advances ( IF 7.9 ) Pub Date : 2021-05-04 , DOI: 10.1016/j.msec.2021.112164 Meili Shen , Hongli Li , Shunyu Yao , Xiaodong Wu , Shun Liu , Qingbiao Yang , Yanjiao Zhang , Jianshi Du , Shaolong Qi , Yapeng Li
|
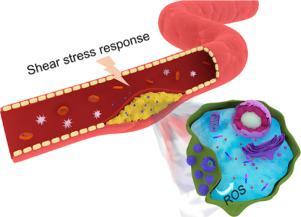
Reactive oxygen species (ROS) are well-known important initiating factors required for atherosclerosis formation, which leads to endothelial dysfunction and plaque formation. Most of the existing antithrombotic therapies use ROS-responsive drug delivery systems, which have a certain therapeutic effect but cannot eliminate excess ROS. Therefore, the atherosclerosis cannot be treated from the source. Moreover, nanoparticles are easily cleared by the immune system during blood circulation, which is not conducive to long-term circulation. In this study, we developed an intelligent response system that could simultaneously respond to ROS and the shear stress microenvironment of atherosclerotic plaques. This system was formed by red blood cells (RBCs) and simvastatin-loaded micelles (SV MC). The micelles consisted of poly(glycidyl methacrylate)-polypropylene sulfide (PGED-PPS). The hydrophobic PPS could react with excess ROS to become hydrophilic, which forced the micelle rupture, resulting in drug release. Most importantly, PPS could also significantly deplete the ROS level, realizing the synergistic treatment of atherosclerosis with drugs and materials. The positively charged SV MC and negatively charged RBCs were self-assembled through electrostatic adsorption to obtain SV MC@RBCs. The SV MC@RBCs could respond to the high shear stress at the atherosclerotic plaque, and the shear stress induced SV MC desorption from the RBC surface. Using biomimetic methods to evade the SV MC@RBCs elimination by the immune system and to reduce the ROS plays a vital role in improving atherosclerosis treatment. The results of in vitro and in vivo experiments showed that SV MC@RBCs could effectively treat atherosclerosis. Moreover, not only does the SV MC@RBCs system avoid the risk of bleeding, but it also has excellent in vivo safety. The study results indicate that the SV MC@RBCs system is a promising therapeutic nanomedicine for treating ROS-related diseases.
中文翻译:

通过消耗ROS剪切应力和ROS响应仿生胶束用于动脉粥样硬化
活性氧(ROS)是形成动脉粥样硬化所需的重要重要启动因子,动脉粥样硬化会导致内皮功能障碍和斑块形成。现有的大多数抗血栓治疗方法都使用ROS反应药物递送系统,该系统具有一定的治疗作用,但不能消除过量的ROS。因此,不能从源头上治疗动脉粥样硬化。而且,纳米颗粒在血液循环期间容易被免疫系统清除,这不利于长期循环。在这项研究中,我们开发了可以同时响应ROS和动脉粥样硬化斑块的切应力微环境的智能响应系统。该系统由红血球(RBC)和负载辛伐他汀的胶束(SV MC)形成。胶束由聚(甲基丙烯酸缩水甘油酯)-聚丙烯硫醚(PGED-PPS)组成。疏水性PPS可能会与过量的ROS发生反应而变成亲水性,从而迫使胶束破裂,从而导致药物释放。最重要的是,PPS还可以显着消耗ROS水平,从而实现药物和材料的协同治疗动脉粥样硬化。带正电荷的SV MC和带负电荷的RBC通过静电吸附自组装,得到SV MC @ RBC。SV MC @ RBCs可以响应动脉粥样硬化斑块上的高剪切应力,并且剪切应力引起SV MC从RBC表面解吸。使用仿生方法来逃避免疫系统对SV MC @ RBC的消除并减少ROS在改善动脉粥样硬化的治疗中起着至关重要的作用。体内外实验结果表明,SV MC @ RBCs可以有效治疗动脉粥样硬化。此外,SV MC @ RBCs系统不仅避免了出血的风险,而且还具有出色的体内安全性。研究结果表明,SV MC @ RBCs系统是用于治疗ROS相关疾病的有前途的治疗纳米药物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号