Structure ( IF 5.7 ) Pub Date : 2021-05-03 , DOI: 10.1016/j.str.2021.04.007 Laura Troussicot 1 , Björn M Burmann 1 , Mikael Molin 2
|
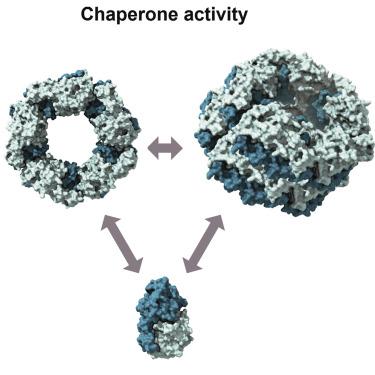
Peroxiredoxins (PRDXs) are abundant peroxidases present in all kingdoms of life. Recently, they have been shown to also carry out additional roles as molecular chaperones. To address this emerging supplementary function, this review focuses on structural studies of 2-Cys PRDX systems exhibiting chaperone activity. We provide a detailed understanding of the current knowledge of structural determinants underlying the chaperone function of PRDXs. Specifically, we describe the mechanisms which may modulate their quaternary structure to facilitate interactions with client proteins and how they are coordinated with the functions of other molecular chaperones. Following an overview of PRDX molecular architecture, we outline structural details of the presently best-characterized peroxiredoxins exhibiting chaperone function and highlight common denominators. Finally, we discuss the remarkable structural similarities between 2-Cys PRDXs, small HSPs, and J-domain-independent Hsp40 holdases in terms of their functions and dynamic equilibria between low- and high-molecular-weight oligomers.
中文翻译:

2-半胱氨酸过氧还蛋白伴侣功能中多聚化和解离的结构决定因素
过氧化物酶 (PRDX) 是存在于所有生命王国中的丰富的过氧化物酶。最近,它们已被证明还可以作为分子伴侣发挥额外的作用。为了解决这一新兴的补充功能,本综述侧重于表现出伴侣活性的 2-Cys PRDX 系统的结构研究。我们提供了对 PRDXs 伴侣功能基础结构决定因素的当前知识的详细了解。具体来说,我们描述了可能调节其四级结构以促进与客户蛋白相互作用的机制,以及它们如何与其他分子伴侣的功能相协调。在概述 PRDX 分子结构之后,我们概述了目前表现出伴侣功能的最典型的过氧化物酶的结构细节,并突出了共同点。最后,我们讨论了 2-Cys PRDX、小 HSP 和独立于 J 域的 Hsp40 保持酶之间在低分子量和高分子量低聚物之间的功能和动态平衡方面的显着结构相似性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号