Chemosphere ( IF 8.8 ) Pub Date : 2021-04-30 , DOI: 10.1016/j.chemosphere.2021.130730 Qiyu Lian 1 , Fahrin Islam 1 , Zaki Uddin Ahmad 2 , Xiaobo Lei 1 , Dilip Depan 3 , Mark Zappi 4 , Daniel D Gang 1 , William Holmes 4 , Hui Yan 5
|
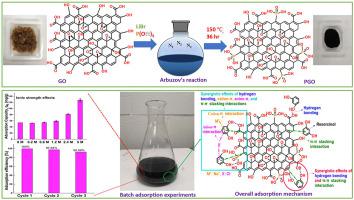
Phosphate functionalized graphene oxide (PGO) was successfully prepared by Arbuzov reaction and employed for adsorption of resorcinol from an aqueous phase. The phosphate functional groups were successfully incorporated onto the PGO surface by the formation of P–C bonds as identified by the analysis of FTIR and XPS spectra. The evaluation of adsorption capacity of resorcinol onto PGO exhibited significant improvement of resorcinol removal, achieving an adsorption capacity of 50.25 mg/g in the pH range of 4–7 which was 15 times higher than pristine graphene oxide. The addition of 2.4 M and 5 M NaCl in the adsorption system significantly increased the adsorption capacity towards resorcinol from 50.25 mg/g to 82.10 mg/g and 128.10 mg/g, respectively. Based on kinetics and adsorption isotherm studies, Pseudo-First-Order and Langmuir model are the best model to describe the adsorption process indicating that the adsorption is dominantly controlled by physisorption. The thermodynamic analysis suggested that the adsorption process was the favorable, spontaneous, and endothermic process. Besides, the interplay of hydrogen bonding and π-π interactions is proposed to be the governing physisorption mechanism. The outstanding reusability and better adsorption performance make PGO a promising adsorbent for environmental remediation of resorcinol.
中文翻译:

间苯二酚对Arbuzov反应合成的磷酸盐官能化氧化石墨烯的增强吸附作用:氢键和π-π相互作用的拟议机理
通过Arbuzov反应成功制备了磷酸功能化氧化石墨烯(PGO),并用于从水相中吸附间苯二酚。通过FTIR和XPS光谱分析可以确定,通过形成P–C键,磷酸盐官能团成功地结合到了PGO表面。对间苯二酚在PGO上的吸附能力进行了评估,结果表明间苯二酚的去除率显着提高,在4–7的pH范围内,间苯二酚的吸附能力为50.25 mg / g,是原始氧化石墨烯的15倍。在吸附系统中添加2.4 M和5 M NaCl可以显着提高对间苯二酚的吸附能力,分别从50.25 mg / g增至82.10 mg / g和128.10 mg / g。根据动力学和吸附等温线研究,伪一阶模型和Langmuir模型是描述吸附过程的最佳模型,表明吸附主要受物理吸附控制。热力学分析表明,吸附过程是有利的,自发的,吸热的过程。此外,氢键与π-π相互作用的相互作用被认为是决定其物理吸附的主要机理。出色的可重复使用性和更好的吸附性能使PGO成为用于间苯二酚环境修复的有前途的吸附剂。氢键和π-π相互作用的相互作用被认为是决定其物理吸附的主要机理。出色的可重复使用性和更好的吸附性能使PGO成为用于间苯二酚环境修复的有前途的吸附剂。氢键和π-π相互作用的相互作用被认为是决定其物理吸附的主要机理。出色的可重复使用性和更好的吸附性能使PGO成为用于间苯二酚环境修复的有前途的吸附剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号